Ferritin-based multi-antigen universal influenza vaccine and preparation method and application thereof
A technology of influenza vaccine and ferritin, applied in the field of ferritin-based multi-antigen universal influenza vaccine and its preparation and application, to achieve broad-spectrum immune protection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0108] A kind of FRT nanoparticle derived from horse spleen (purchased from Sigma-Aldrich Co. LLC., the FRT nanoparticle carrier described in the following experimental examples 2-14 is the same as experimental example 1) as a carrier, the surface of which is covalently bound with M2e antigen The preparation method of antigen influenza vaccine M2e.FRT is as follows:
[0109] 1) Artificial synthesis of M2e polypeptide antigen derived from A / Puerto Rico / 8 / 1934 H1N1 influenza virus (SEQ ID NO. 1).
[0110] 2) Prepare a FRT carrier solution with a final concentration of 1 mg / mL in 0.1M PBS (pH 7.4), and add 10 times the molar amount of NHS-PEG to it n -Mal crosslinking agent, react for 1h in a shaker at 4°C in the dark to modify the amino end of the FRT with maleimide groups, and then take out and remove the unreacted residual crosslinking agent. The 0.5 mg / mL PEG-FRT solution modified by PEG cross-linking agent was mixed with 5 times the molar amount of the FRT subunit M2e polypeptide...
experiment example 2
[0113] A single antigen influenza vaccine NP.FRT with NP antigen distributed on the surface of FRT nanoparticles.
[0114] Compared with Experimental Example 1, in addition to the artificially synthesized NP polypeptide antigen derived from A / Puerto Rico / 8 / 1934H1N1 influenza virus (SEQ ID NO. 2) instead of M2e antigen for covalent cross-linking with FRT, its preparation method Same as experimental example 1. Finally, the NP.FRT single antigen influenza vaccine with NP antigen covalently bound on the surface is prepared, and the covalent binding rate of the surface NP antigen is about 15 NP antigen polypeptide molecules per NP.FRT molecule. (This example serves as a comparative example)
[0115] SEQ ID NO.2: QIASNENMETMESSTL-C
experiment example 3
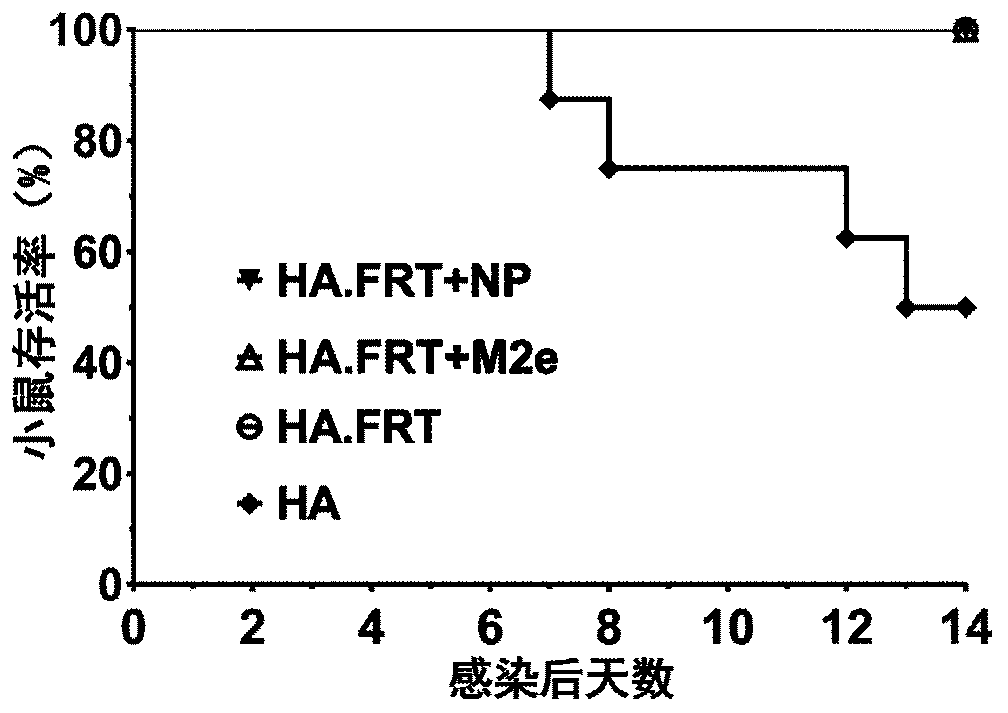
[0117] A single antigen influenza vaccine HA.FRT with HA full-length protein antigen covalently bound to the surface of FRT as a carrier, the preparation method is as follows:
[0118] 1) Recombinant expression of A / Puerto Rico / 8 / 1934H1N1 influenza virus HA full-length protein (SEQ ID NO. 3), and purification, the final purity is more than 90%.
[0119] 2) According to the method described in Experimental Example 1, prepare a FRT carrier solution with a final concentration of 1 mg / mL with 0.1M PBS (pH 7.4), and add 10 times the molar amount of NHS-PEG to it n -Mal crosslinking agent, react for 1h in a shaker at 4°C in the dark to modify the amino end of the FRT with maleimide groups, and then take out and remove the unreacted residual crosslinking agent. PEG-FRT solution modified by PEG cross-linking agent 0.5mg / mL, the same as 2 times the molar amount of FRT tris(2-carboxyethyl)phosphine-hydrochloride (TCEP·HCl) reduction treatment of recombinant HA antigen Mix and react on a shak...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com