Electrochemical preparation method of beta-trifluoromethylamide compounds
A technology of trifluoromethyl amide and trifluoromethyl, which is applied in the direction of electrodes, electrolysis process, electrolysis components, etc., can solve the problems of cumbersome preparation steps of three-step methylation reagents, expensive photocatalytic catalysts, and hindering the development of applications. , to achieve good functional group tolerance, less waste emissions, and avoid the use of catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
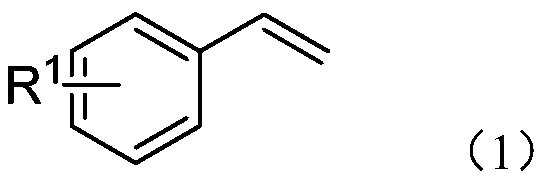
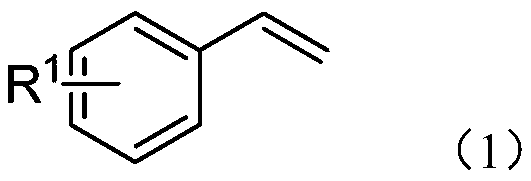
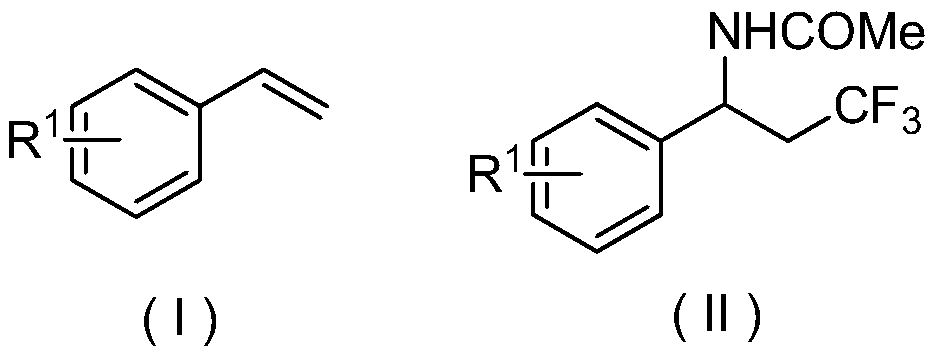
[0025] Embodiment 1 of the present invention is: an electrochemical preparation method of β-trifluoromethyl amide compounds, comprising the following steps: using aryl acetylene compounds represented by formula (I) as raw materials, trifluoromethyl trifluoromethyl Fluorosilane is used as a source of trifluoromethyl, and by electrochemical anodic oxidation, tetra-n-butylammonium acetate is used as an electrolyte, reacted in a solvent composed of acetonitrile and ethanol for a period of time, and then purified to obtain formula (II) The β-trifluoromethylamide product shown;
[0026]
[0027] In the scheme of the present invention, with CF 3 SO 2 Na is used as a trifluoromethyl source, acetonitrile is used as both a reactant and a solvent, and a cyano group is used as an amide source to achieve trifluoromethylation of the amino group at the ortho position; trifluoromethyltrifluorosilane is used as a trifluoromethyl The base source and tetra-n-butylammonium acetate are used a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com