Systems and methods for modeling disease and assessing adverse side effects of therapeutics therefor
A compound, anisotropic technology, used in the field of in vitro analysis of cardiotoxicity, which can solve problems such as inability to detect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0044] hESC culture, directed cardiac differentiation and hvCAS formation
[0045] In feeder-free and serum-free mTeSR supplemented with hESC-specific Matrigel™ (354277; BD Biosciences) at 37°C TM 1 conditions (Stemcell Technologies, Inc.), with 5% CO 2 The human embryonic cell (hESC) line HES2 (ESI, NIH code ES02) was maintained in its pluripotent state in a balanced humidified normoxic incubator. Directed cardiac differentiation of hESC cultures was obtained according to an established protocol that efficiently generates ventricular (V) subtype cardiac cells with high yield and purity [18] .
[0046] hESC cultures at 80% to 90% confluence were dissociated into single cells with accutase (A11105; Gibco) and subsequently plated in ultra-low attachment plates (3471; Corning) in hypoxia (5% o 2 / 5%CO 2 ) in suspension for 8 days. Within the first 24 hours mTeSR-based therapy supplemented with Rho kinase (ROCK) inhibitor (1254; R&D), bone morphogenetic protein-4 (BMP4; PHC9...
example 2
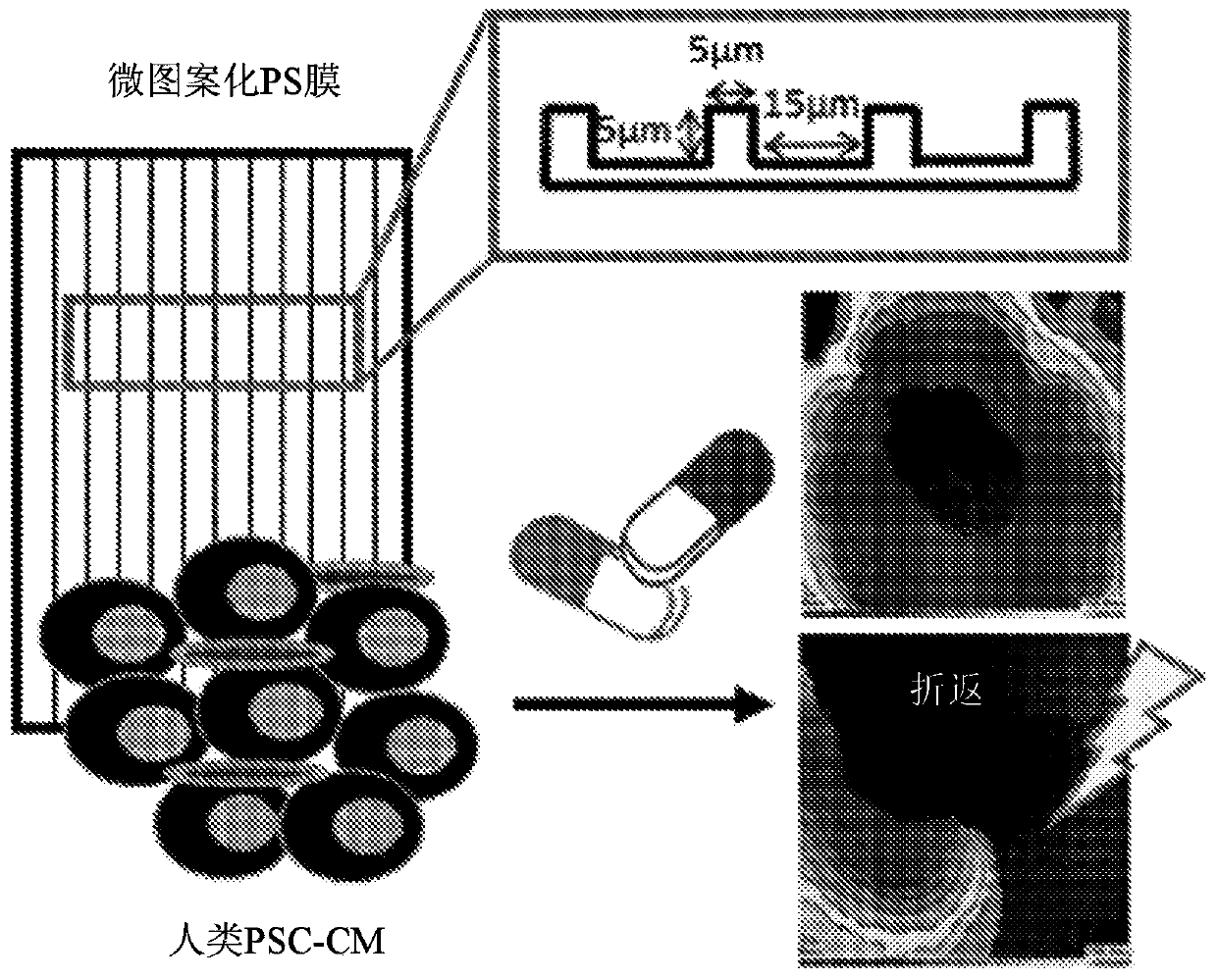
[0048] Fabrication of Microgroove Substrates
[0049] Photolithography was used to generate polydimethylsiloxane (PDMS) molds to create 10 μm (R) × 5 μm (D) × 5 μm (W) or 15 μm (R) × 5 μm (D) × 5 μm (W) L10 or L15. Then, the microscopic line features were hot embossed onto polystyrene (PS) shrink film (Clear Shrink )superior. Substrates were then UVO-treated for 8 minutes (Jetlight UVO) and finally sterilized by immersion in 70% ethanol, followed by UVO treatment for at least 20 minutes before use.
example 3
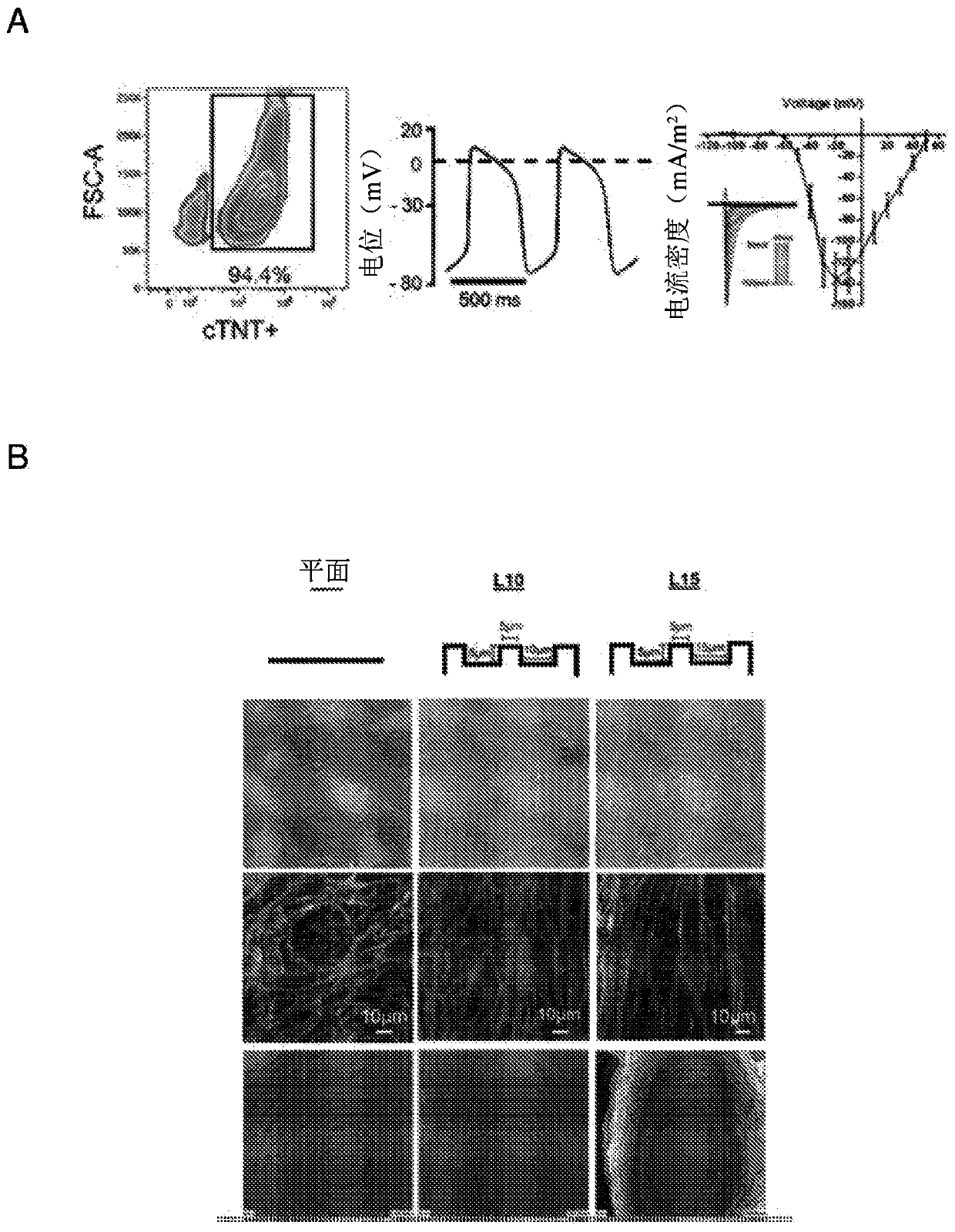
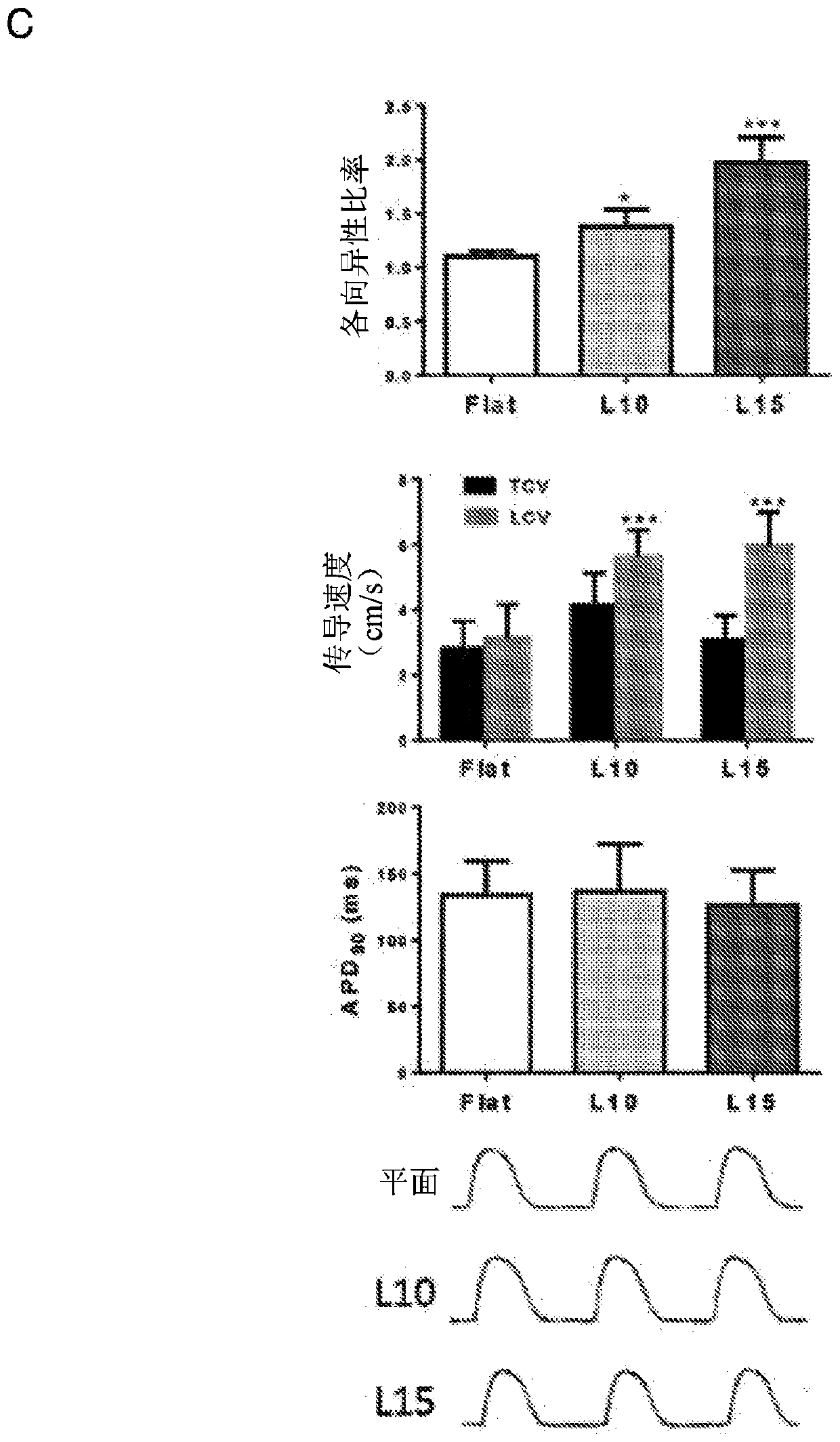
[0051] Optical mapping and electrophysiology of hvCAS
[0052] Di-8-ANEPPS (10 μM; D-3167; Molecular Probes) / Pluronic F-12 (0.04%; P-3000MP; Life Technologies (Life Technologies) Technologies)) hvCAS preparations were loaded in DMEM-F12 and incubated in Blebistatin (50 μM; B0560; Sigma-Aldrich) in Tyrode's solution for 15 min at room temperature (RT). Benchtop solution consists of 140mM NaCl, 5mM KCl, 1mM MgCl 2 , 1mM CaCl 2 , 10 mM D-glucose, and 10 mM HEPES at pH 7.4 to minimize the potential interference of motion artifacts on the optical mapping signal. Dye-loaded hvCAS preparations were soaked in a benchtop solution maintained at 35°C to 37°C using a Petri dish incubator (Warners Instruments). High resolution optical mapping of AP and transmission properties was performed using a Micam Ultima (SciMedia, CA, USA) with a 1x objective lens and a 1x condenser lens for a field of view of 10mm*10mm. Fluorescent imaging was performed using a halogen lamp filtered through a 5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Width | aaaaa | aaaaa |
| Width | aaaaa | aaaaa |
| Width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap