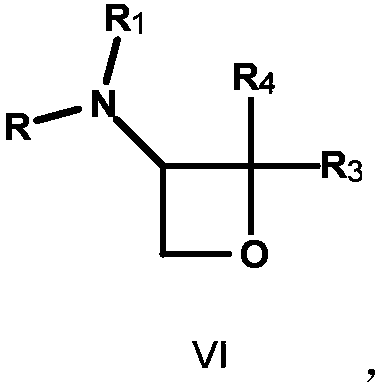
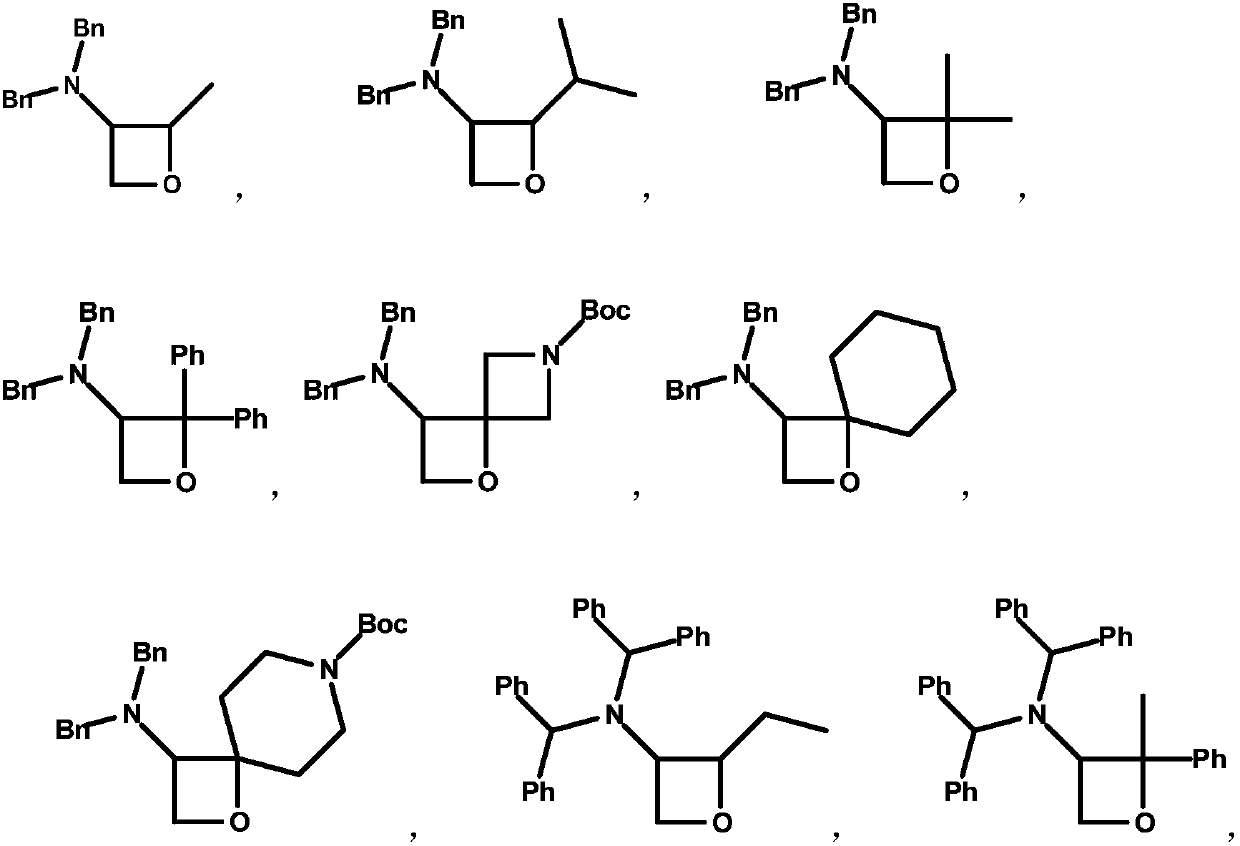
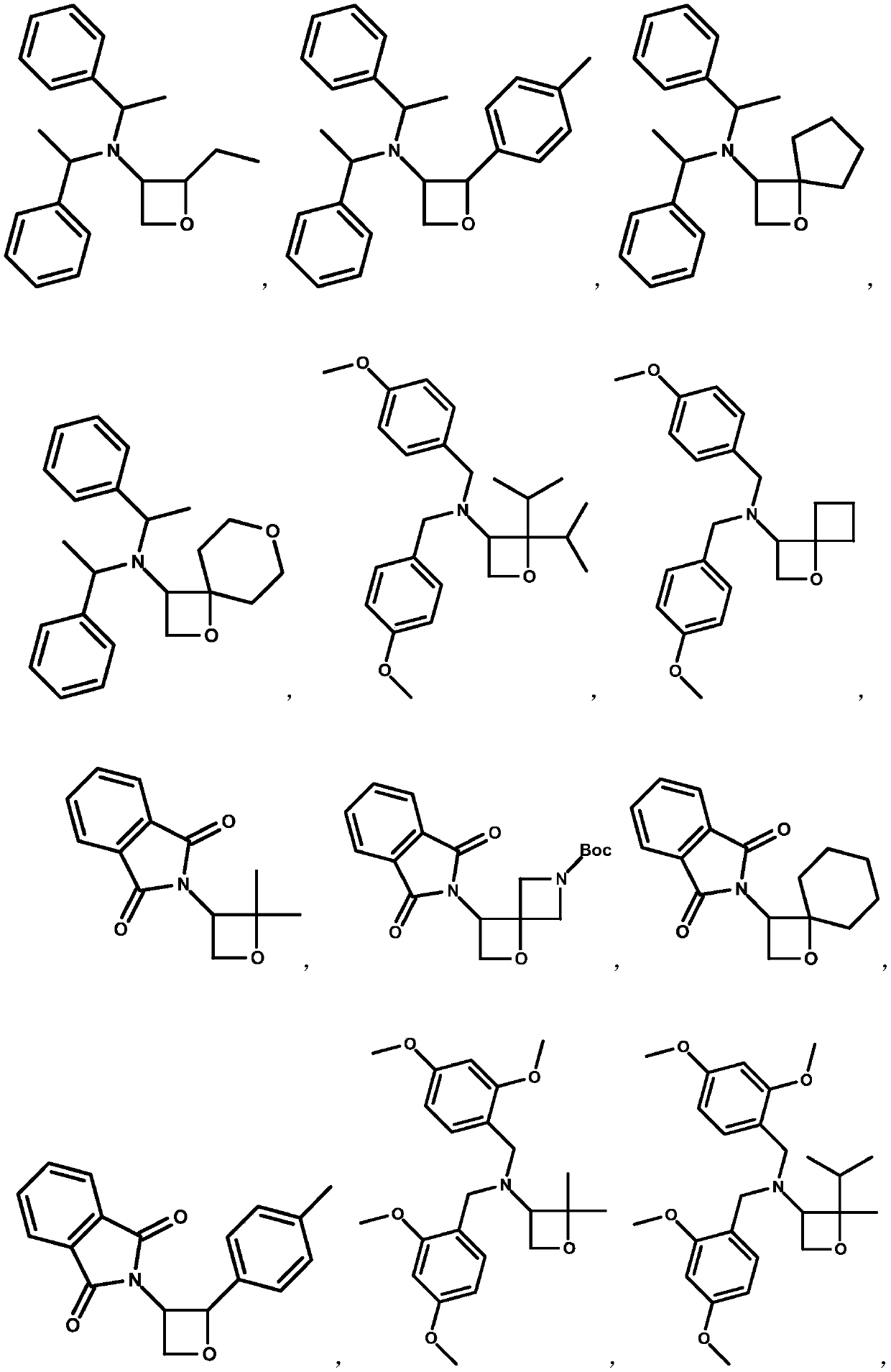
3-amino-oxetane derivative as well as preparation method and application thereof
A technology of oxetane and its derivatives, which is applied in the field of 3-amino-oxetane derivatives and its preparation, can solve the problems of not easy to obtain raw materials, long synthetic route, high cost, etc., and achieve market supply Sufficient, short steps, the effect of increasing the success rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076]
[0077] Preparation of Compound IV-1:
[0078] Dissolve diisopropylamine (56.0g, 0.55mol, 1.25eq.) in THF, cool to -78°C, add n-butyllithium (212mL, 0.53mol, 1.2eq.) dropwise, and react for 30min after the addition is complete , and the prepared LDA solution was set aside.
[0079] Compound II-1 (125.0g, 0.44mol, 1.0eq.) was dissolved in THF (2L), and the above-mentioned self-made LDA solution was added dropwise at -78°C. After the dropwise addition, the reaction was carried out for 30min, and acetone (31.0g, 0.53mol, 1.2eq.), reacted for 2h, poured into saturated ammonium chloride solution (1L), separated into layers, dried and concentrated the organic phase, added n-heptane (100mL), stirred in an ice-water bath, and solid precipitated out, filtered , the solid was dried to obtain 81.0 g of white solid, yield: 54.8%. 1 H-NMR (400MHz, CDCl 3 )δ (ppm) 7.25-7.41 (m, 10H), 4.30-4.44 (m, 2H), 4.23-4.28 (m, 2H), 3.41-3.47 (m, 3H), 3.15 (s, 1H), 1.46- 1.48 (m, 3H), 1....
Embodiment 2
[0085]
[0086] Preparation of Compound IV-2:
[0087] Compound II-2 (125.0g, 0.364mol, 1.0eq.) was dissolved in THF (2L), and a THF solution of 1M LiHMDS (364mL, 0.364mol, 1.0eq.) was added dropwise at -78°C. After the addition was complete, , reacted for 30min, and added compound III-2 (41.56g, 0.364mol, 1.0eq.) dropwise at -78°C. After the addition, the temperature was naturally raised to -5°C, and the reaction was stirred for 2h. LC-MS detection showed that the reaction of the raw materials was complete. The liquid was poured into saturated ammonium chloride solution (1L), separated into layers, the organic phase was dried and concentrated, added n-heptane (100mL), stirred in an ice-water bath, a solid precipitated out, filtered, and the solid was dried to obtain compound IV-2 as a white solid 111.2 g, yield: 64.8%.
[0088] Preparation of Compound V-2:
[0089] Compound IV-2 (80.0g, 0.170mol, 1.0eq.), was dissolved in 600mL of dioxane, under ice-cooling, NaBH was add...
Embodiment 3
[0093]
[0094] Preparation of compound IV-3:
[0095] Compound II-3 (37.70g, 0.14mol, 1.0eq.) was dissolved in dioxane, under the protection of nitrogen, the temperature was lowered to -80°C, and 1M 2,2,6,6-tetramethylpiperidine was added dropwise After the THF solution of lithium (420mL, 0.42mol, 3.0eq.) was added dropwise, the reaction was incubated for 30min, and compound III-3 (30.28g, 0.42mol, 3.0eq.) was added dropwise, and the reaction was stirred at -50°C for 2h. TLC showed that the raw material disappeared, the reaction solution was poured into 300mL of saturated ammonium chloride, separated, the organic phase was dried, and concentrated to obtain a yellow oil, which was added to 100mL of n-heptane and stirred to precipitate a solid, which was filtered to obtain compound IV-3 as white The solid was 28.68g, and the yield was 60%. 1 HNMR (400MHz, CDCl 3 )δ (ppm): 7.26-7.35 (m, 10H), 4.28-4.43 (m, 2H), 3.86-3.93 (m, 3H), 3.43-3.47 (d, 2H), 3.32-3.35 (d, 1H) , 2.13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com