Amide-containing benzimidazole compounds and applications thereof
A technology of benzimidazole and compound, applied in the field of T-LAK cell-derived protein kinase inhibitor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
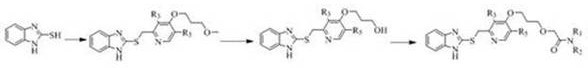
[0059] The schemes outline the preparative steps used to prepare the compounds of the invention.
[0060]
[0061] process
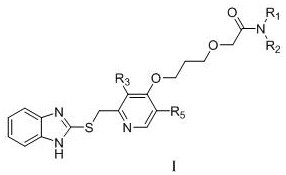
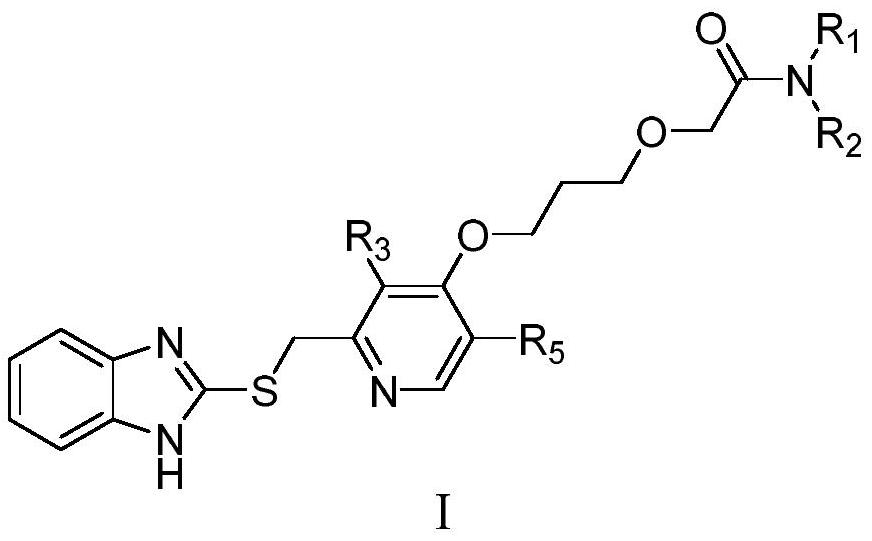
[0062] Among them, R 1 , R 2 , R 3 , R 5 as mentioned earlier.
[0063] The present invention is described in detail with the following examples. However, it should be understood that the present invention is not limited to the specific recited examples below.
Embodiment 1
[0064] Example 1: 2-{3-{{2-{[(1H-benzimidazol-2-yl)thio]methyl}-3-methylpyridin-4-yl}oxy}propoxy} -Preparation of N-cyclohexylacetamide (compound X01)
[0065] Step A: Preparation of 2-{[4-(3-methoxypropoxy)-3-methylpyridin-2-yl]methylthio}-1H-benzimidazole
[0066] 2-Chloromethyl-4-(3-methoxypropoxy)-3-picoline hydrochloride (0.50g, 1.88mmol) was placed in a 125ml eggplant-shaped bottle, and 11ml of ethanol was added to dissolve it, Then 1H-benzimidazole-2-thiol (0.28g, 1.88mmol) and 4ml NaOH (80g / L) were added, refluxed at 68°C for 4h, and the reaction was complete as monitored by TLC. The reaction liquid was poured into a 100ml beaker, cooled naturally to room temperature, a white solid was precipitated, and recrystallized from ethyl acetate and petroleum ether (2:1) to obtain 0.58 g of white needle-like crystals, with a yield of 89.3%. m.p.: 115-118°C (literature value: 116-118°C).
[0067] Step B: Preparation of 3-{{2-{[(1H-benzimidazol-2-yl)thio]methyl}-3-methylpyridi...
Embodiment 2
[0071] Example 2: 2-{3-{{2-{[(1H-benzimidazol-2-yl)thio]methyl}-3-methylpyridin-4-yl}oxy}propoxy} -Preparation of N-isopropylacetamide (compound X02)
[0072] Referring to the preparation method of Example 1, 0.57 g of white solid was obtained with a yield of 87.2%. m.p.: 162.9-164.7℃; IR: (KBr,cm -1 )3278,3086,2924,1656,1584,1464,1442,1369,732; 1 H-NMR (400MHz, DMSO): δ8.36(d, 1H, J=7.4Hz, Pyridine-6-H), 8.22(d, 1H, J=5.6Hz, Ar-H), 7.63~7.45(m ,1H,Ar-H),7.44~7.37(m,1H,Ar-H),7.21~7.13(m,2H,Pyridine-5-H,N-H),6.96(d,1H,J=5.7Hz,Ar -H),4.78(s,2H,SCH 2 ),4.69(s,2H,OCH 2 ), 4.12(t, J=6.2Hz, 2H, CH 2 ),3.82(dq,J=13.5,6.7Hz,1H,NCH),3.57(dd,J=10.5,5.7Hz,2H,CH 2 ),2.20(s,3H),1.89(p,J=6.1Hz,2H),1.08(s,3H),1.08(s,3H),1.07(s,3H); ESI-MS(m / z) :429.3([M+H] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com