A tin-based fluoride msnf 4 Preparation method of room temperature solid fluoride ion battery with layered fluoride ion electrolyte
A technology of fluoride ions and fluorides, applied in battery electrodes, secondary batteries, circuits, etc., to achieve the effects of simple preparation process, simplified preparation process, and high working temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
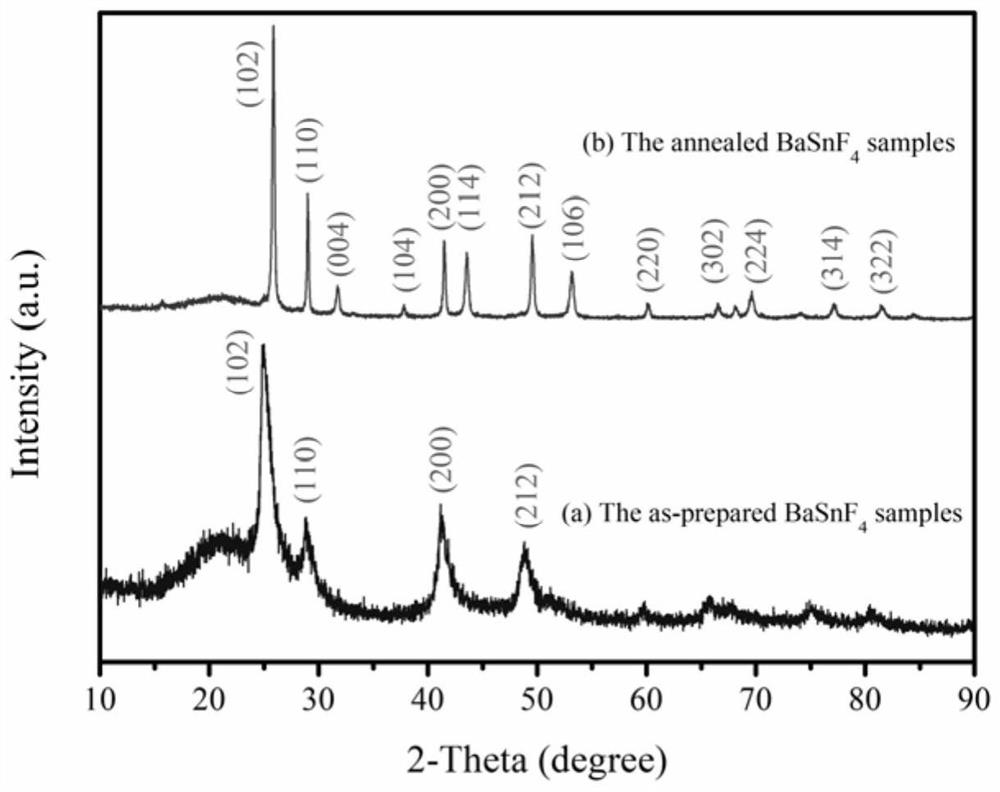
[0028] Weigh 5.2805g BaF respectively 2 and 4.7195g SnF 2 , Under the protection of argon, the ball milling speed is 600rpm, the ball milling time is 20h, and the obtained ball milling product is dried at 80°C for 10h. figure 1 (a) BaSnF prepared by high-energy ball milling in this example 4 XRD pattern of the precursor powder. The obtained dried product was sintered under the protection of nitrogen gas, the sintering temperature was 300 °C, and the heating rate was 6 °C min -1 , the sintering time was 3h, and BaSnF was obtained 4 Electrolyte powder. figure 1 BaSnF prepared before and after sintering in this example 4XRD pattern of the electrolyte powder. BaSnF before sintering 4 Electrolyte powder XRD ( figure 1 (a)) represent (102), (110), (200) and (212) characteristic peaks at 2θ=24.84°, 28.88°, 41.44° and 48.91°, respectively, which indicate the BaSnF before sintering 4 The electrolyte powder has a cubic phase structure. BaSnF after sintering 4 Electrolyte powd...
Embodiment 2
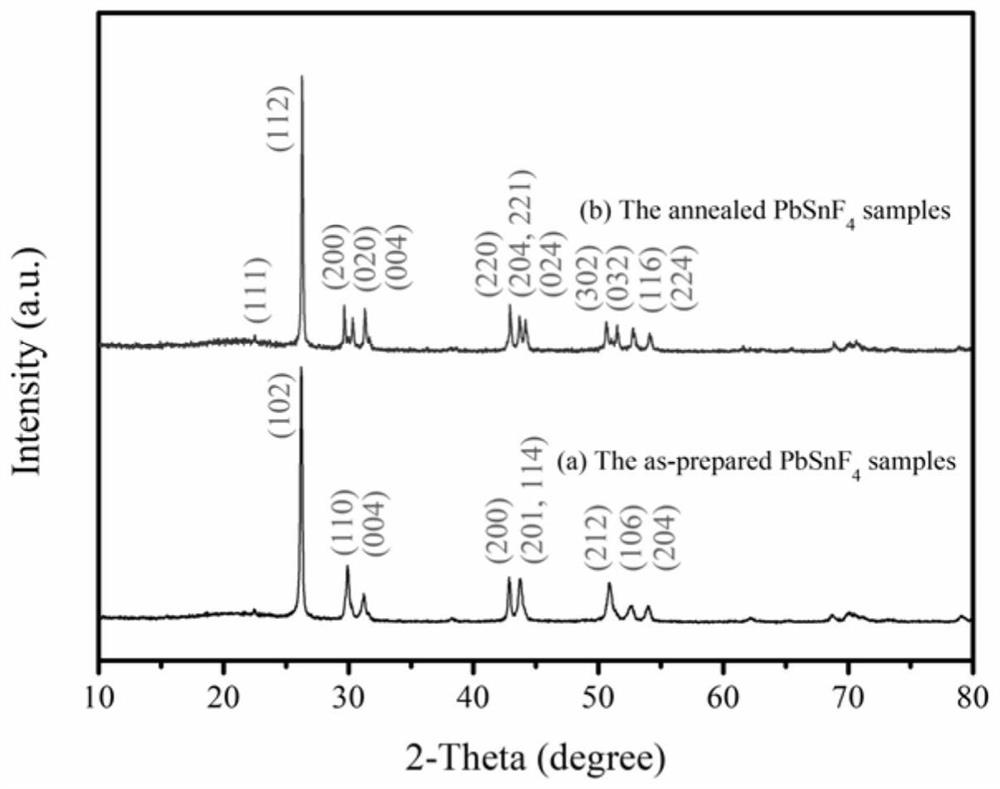
[0030] Weigh 6.0889g PbF respectively 2 and 3.9112g SnF 2 , Under nitrogen protection, the ball milling speed is 800rpm, the ball milling time is 15h, and the obtained ball milling product is dried at 60°C for 14h. figure 2 (a) PbSnF prepared by high-energy ball milling in this example 4 XRD pattern of the precursor powder. The obtained dried product was sintered under the protection of argon gas, the sintering temperature was 450 °C, and the heating rate was 3 °C min -1 , the sintering time is 2h, and the PbSnF 4 Electrolyte powder. figure 2 (b) PbSnF prepared after sintering in this example 4 XRD pattern of the electrolyte powder. PbSnF before and after sintering 4 XRD patterns of electrolyte powder and BaSnF 4 The same characteristic peak differentiation of the electrolyte powder, by comparison with the standard card, indicates that PbSnF 4 The electrolyte powder is a tetragonal phase before sintering, and an orthorhombic phase after sintering. image 3 (e) PbSn...
Embodiment 3
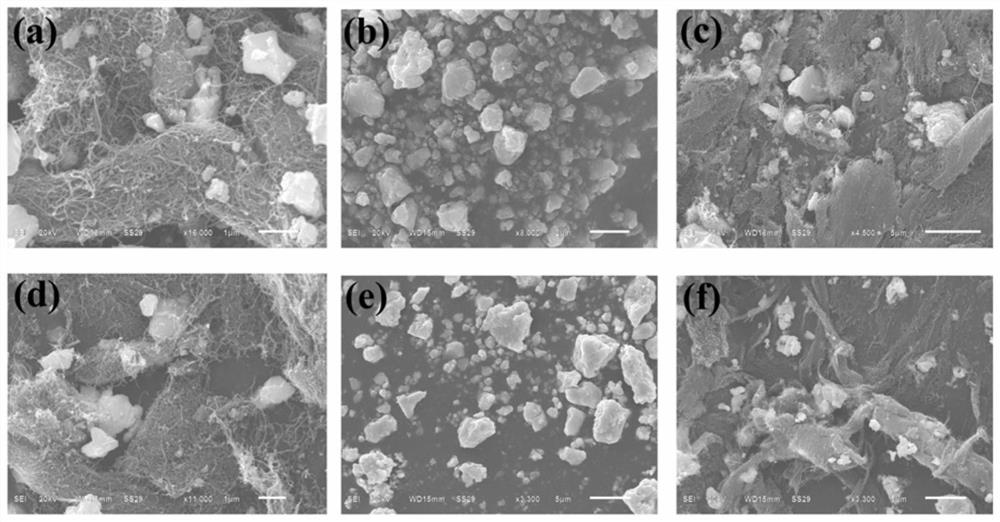
[0032] Weigh 0.8g BiF respectively 3 , 0.2g carbon nanotubes and 1.0g BaSnF 4 or PbSnF 4 , under the protection of nitrogen, the ball milling speed is 200rpm, the ball milling time is 2h, and the obtained ball milling product is dried at 60 ℃ for 3h to prepare BiF 3 Composite cathode material. in image 3 (a) BiF of this embodiment 3 / BaSnF 4 / SEM image of carbon nanotube composite cathode material, image 3 (d) BiF of this embodiment 3 / PbSnF 4 SEM image of carbon nanotube composite cathode material. The SEM image shows that the electrolyte, electrode material and carbon nanotubes are uniformly distributed. Weigh 1.0g Sn, 0.2g carbon nanotubes and 0.8g BaSnF respectively 4 or PbSnF 4 , under the protection of argon, the ball milling speed is 150rpm, the ball milling time is 4h, the obtained ball milling product is dried at 70℃ for 2h, and the Sn composite negative electrode material is prepared. in image 3 (c) Sn / BaSnF of this embodiment 4 / SEM image of carbo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com