Application of canagliflozin in preparation of anti-tumor medicines
An anti-tumor drug and drug technology, applied in anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems of slow progress and retention of small molecule drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Canagliflozin significantly down-regulated the PD-L1 protein level after 24 hours of treatment on non-small cell lung cancer H1299 cells and osteosarcoma MG63 cells. Specific steps are as follows:
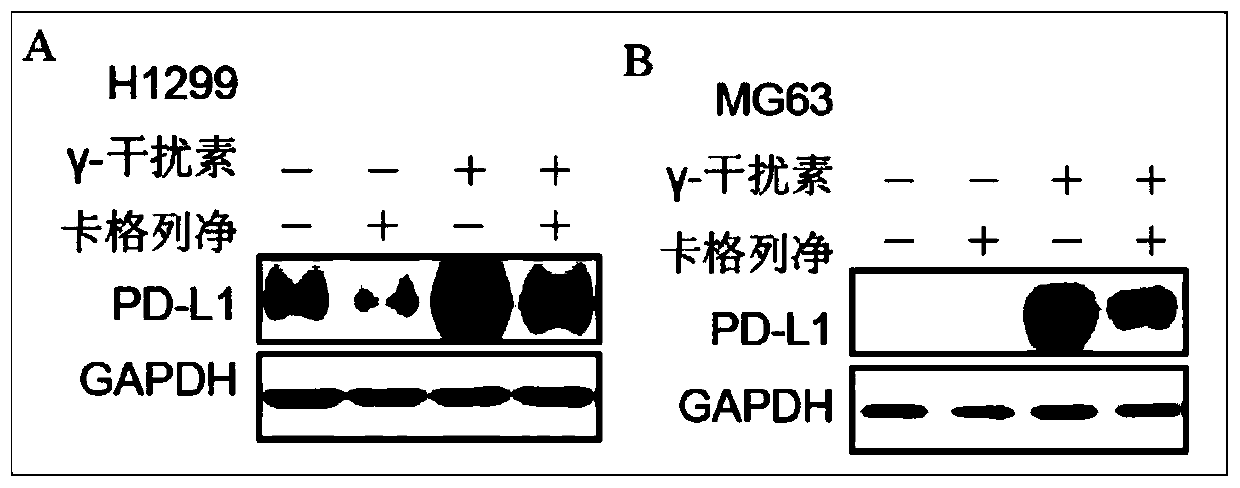
[0017] Multiple strains of human tumor cells were selected from non-small cell lung cancer H1299 cells and osteosarcoma MG63 cells to inoculate in 6-well plates, 1.0×10 6 Cells / well, 37°C, 5% CO 2 Cultured in the incubator overnight; the next day, canagliflozin (Canagliflozin, 20μM) and γ-interferon (IFNγ, 10ng / ml) were administered for 24 hours, the cells were collected and lysed, the protein was extracted, and the PD- in the cells was detected by Western blotting. For the expression of L1, the inhibitory effect of specific concentrations of canagliflozin on PD-L1 protein in non-small cell lung cancer H1299 cells and osteosarcoma MG63 cells can be found in figure 1 , Canagliflozin can significantly inhibit the total protein expression of PD-L1 in tumor cells.
Embodiment 2
[0019] Canagliflozin significantly down-regulated the level of PD-L1 protein on the cell surface after 24 hours of treatment on non-small cell lung cancer H1299 cells and osteosarcoma MG63 cells. Specific steps are as follows:
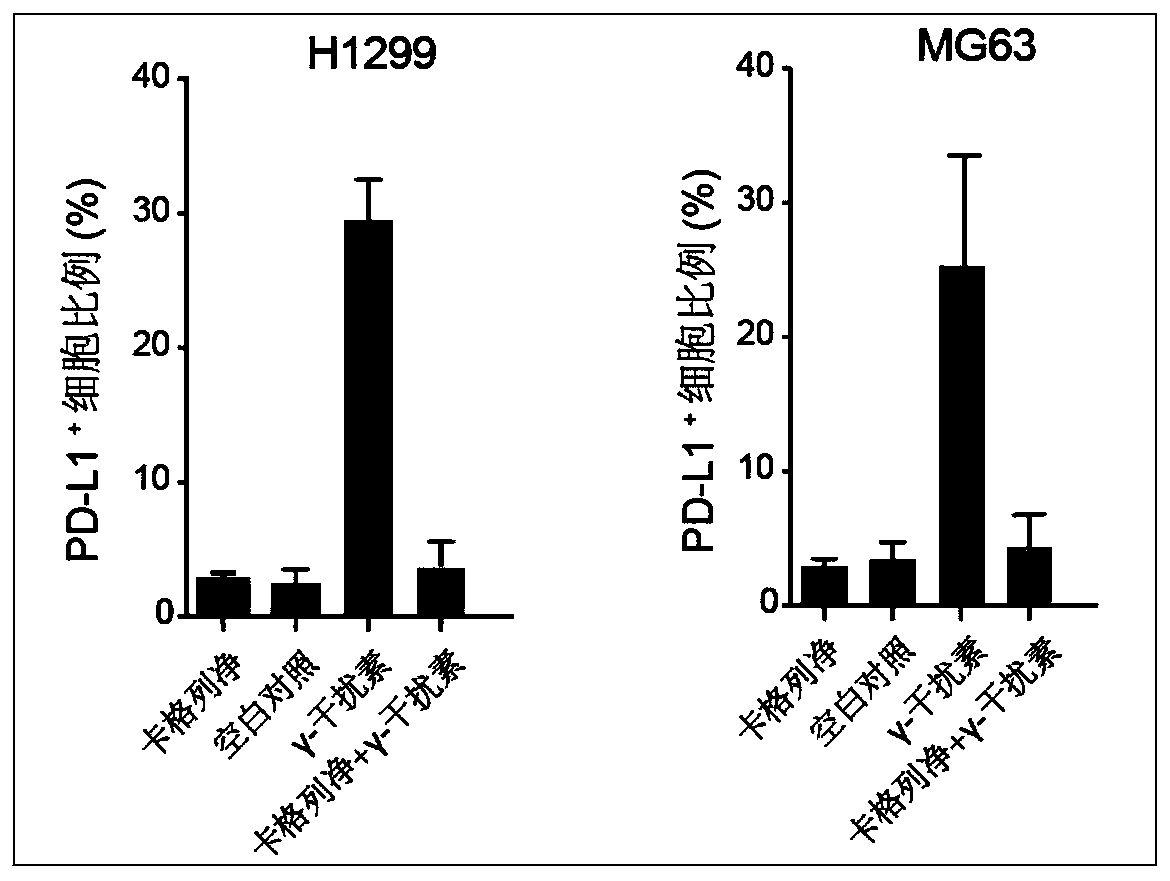
[0020] Two lines of human tumor cells, non-small cell lung cancer H1299 cells and osteosarcoma MG63 cells, were selected and inoculated in 6-well plates, 1.0×10 6 Cells / well, 37°C, 5% CO 2 Cultured in the incubator overnight; the next day, canagliflozin (Canagliflozin, 20 μM) and γ-interferon (IFNγ, 10 ng / ml) were administered for 24 hours, and flow cytometry was used to detect the expression of PD-L1 on the cell surface. Inhibitory effect of canagliflozin on PD-L1 protein in non-small cell lung cancer H1299 cells and osteosarcoma MG63 cells figure 2 , Canagliflozin can significantly inhibit the expression of PD-L1 protein on the surface of tumor cells.
Embodiment 3
[0022] Effect of canagliflozin on the stability of PD-L1 protein in non-small cell lung cancer H1299 cells. Specific steps are as follows:
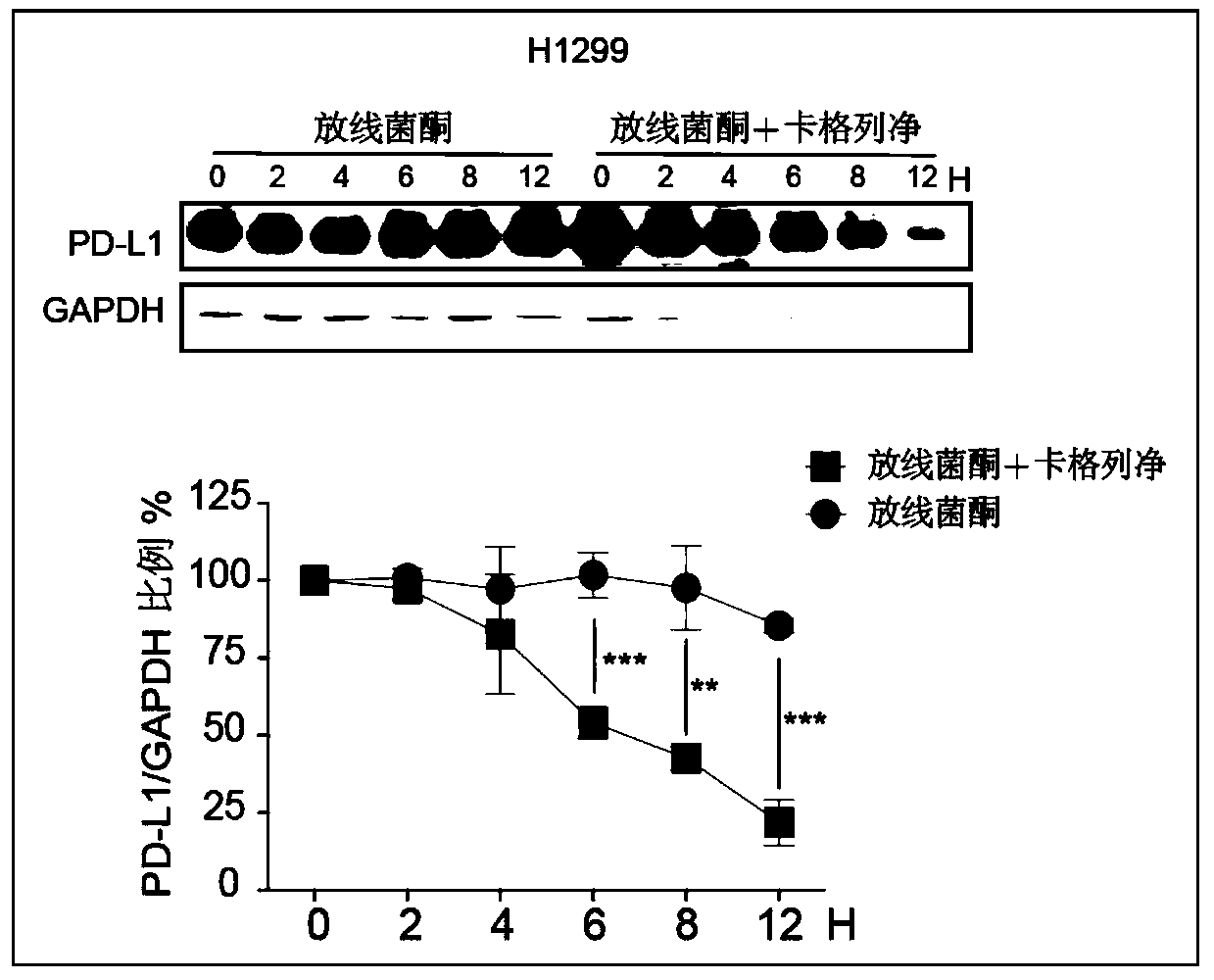
[0023] Non-small cell lung cancer H1299 cells were selected and seeded in 6-well plates, 1.0×10 6 Cells / well, 37°C, 5% CO 2 Incubate overnight in the incubator. The next day, IFNγ (10ng / ml) was administered for 24 hours in advance, and then the protein synthesis inhibitor cycloheximide (CHX, 10μg / mL) or CHX (10μg / mL) + Canagliflozin (20μM) co-administration groups were administered at the same time. At 0h, 2h, 4h, 6h, 8h, and 12h, the cells were collected, and the protein level of PD-L1 in the cells was detected by Western blotting, grayscale analysis was performed, and the stability of the protein was investigated after a graph was drawn. See the specific role image 3 , Canagliflozin can significantly promote the degradation of PD-L1 protein and shorten its protein half-life.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com