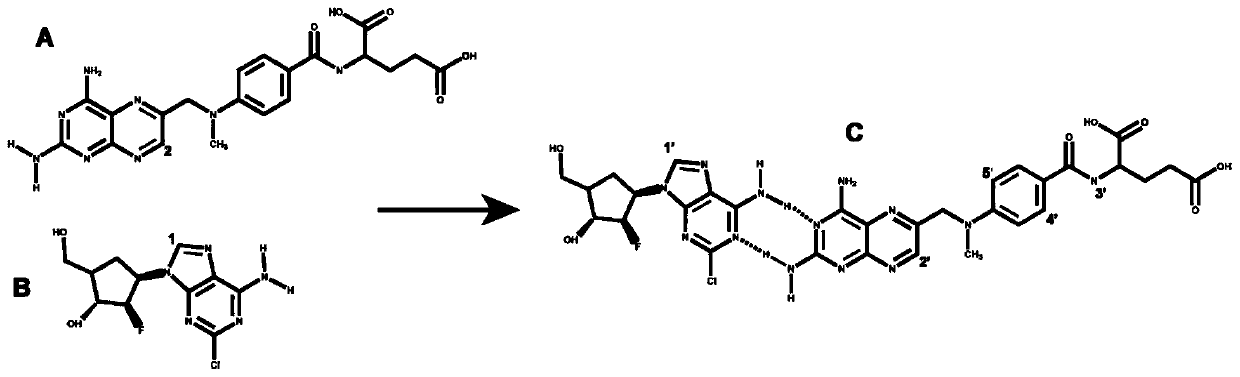
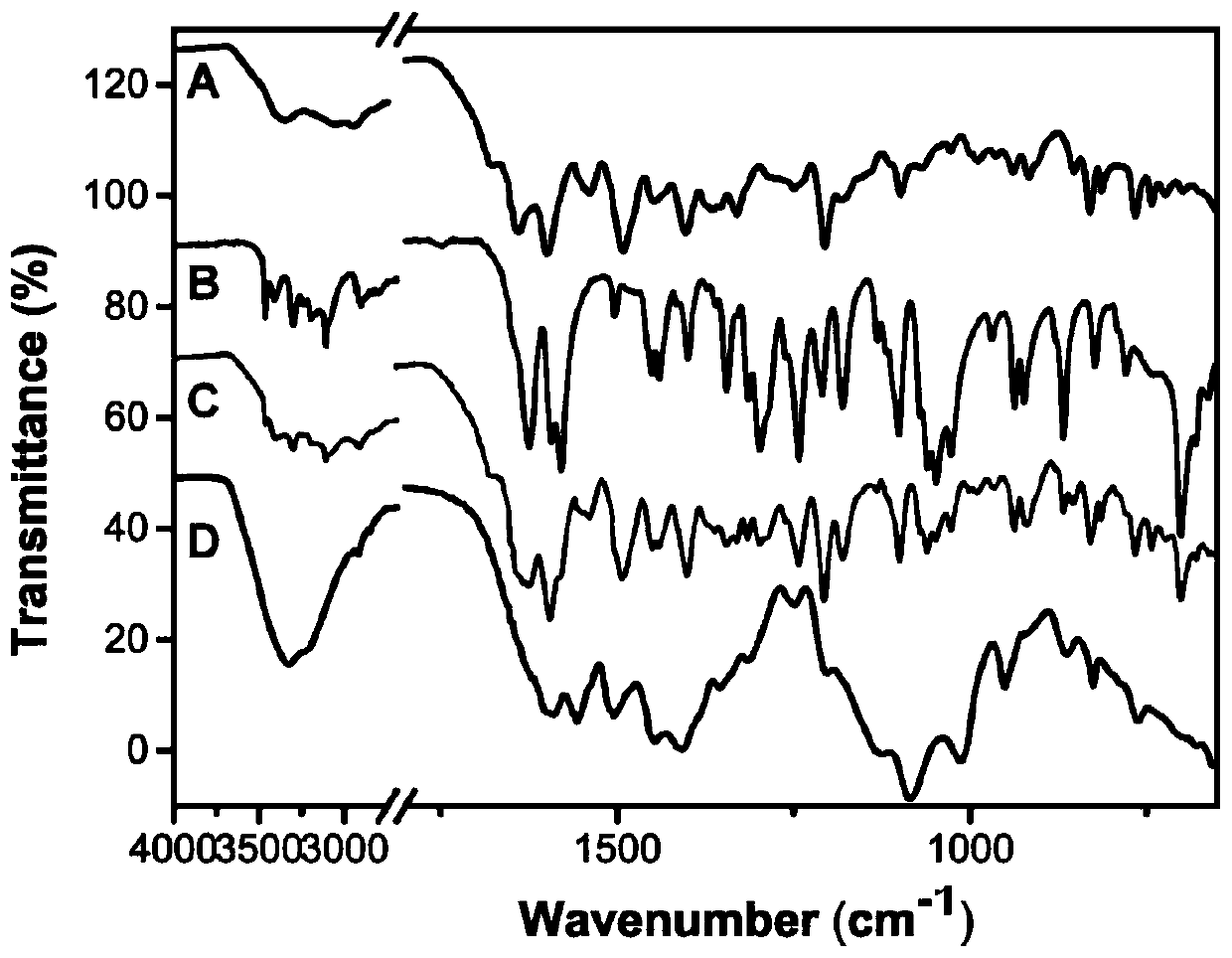
Clofarabine and methotrexate double-medicine preparation and preparation method thereof
A technology of clofarabine methotrexate and clofarabine, which is applied in the field of clofarabine methotrexate double-drug preparation and its preparation, and achieves the effects of good stability, good responsive release characteristics, and strong tumor inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Clofarabine and methotrexate were dissolved in dimethyl sulfoxide respectively to obtain 2 mg / mL clofarabine solution and 3 mg / mL methotrexate solution;
[0031] (2) Mix 500 μL clofarabine solution and 500 μL methotrexate solution (molar ratio 1:1) and react at 25° C. for 0.5 h;
[0032] (3) Add 10 μL of sodium hydroxide solution with a concentration of 5 mg / mL to the material obtained in step (2), and continue to react at 25° C. for 0.5 h;
[0033] (4) In step (3), add 10 times the volume of deionized water for super-dispersion for 0.5 h, and then use a dialysis bag with a molecular weight of 3500 to perform dialysis for 12 h to remove dimethyl sulfoxide to obtain a dialysate;
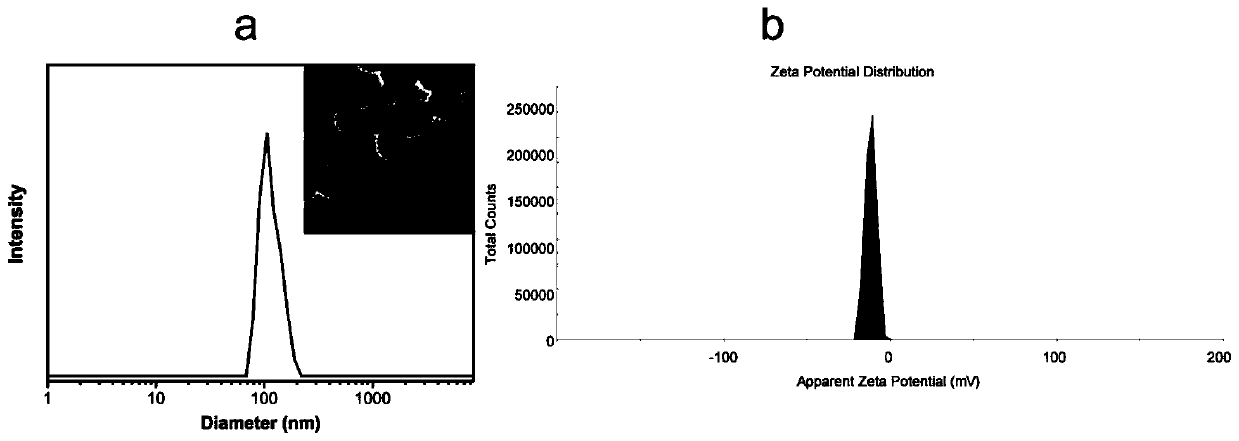
[0034] (5) Filter the dialysate with a microporous membrane of 0.22 μm to obtain a clofarabine methotrexate double-drug nanomicelle solution with an average particle diameter of 110 nm;
[0035] (6) Add 5wt% dextran to the above-mentioned clofarabine methotrexate double-drug nano-micelle s...
Embodiment 2
[0037] (1) Clofarabine and methotrexate were dissolved in N,N-dimethylformamide respectively to obtain 2mg / mL clofarabine solution and 3mg / mL methotrexate solution;
[0038] (2) Mix 500 μL clofarabine solution and 500 μL methotrexate solution (molar ratio 1:1) and react at 30° C. for 0.5 h;
[0039] (3) Add 10 μL of sodium hydroxide solution with a concentration of 5 mg / mL to the material obtained in step (2), and continue to react at 30° C. for 0.5 h;
[0040] (4) Add 10 times the volume of deionized water in step (3) and ultrasonically disperse for 0.5 h, then use a dialysis bag with a molecular weight of 3500 to perform dialysis for 12 h to remove N, N-dimethylformamide to obtain a dialysate;
[0041] (5) Filter the dialysate with a microporous membrane of 0.22 μm to obtain a clofarabine methotrexate double-drug nanomicelle solution with an average particle diameter of 110 nm;
[0042] (6) Add the clofarabine methotrexate double-drug nano-micelle solution to the lyoprotect...
Embodiment 3
[0044] (1) Clofarabine and methotrexate were dissolved in tetrahydrofuran respectively to obtain 2mg / mL clofarabine solution and 3mg / mL methotrexate solution;
[0045] (2) Mix 500 μL clofarabine solution and 500 μL methotrexate solution (molar ratio 1:1) and react at 25° C. for 0.5 h;
[0046] (3) Add 10 μL of sodium hydroxide solution with a concentration of 5 mg / mL to the material obtained in step (2), and continue to react at 25° C. for 0.5 h;
[0047] (4) In step (3), add 10 times the volume of deionized water to ultrasonically disperse for 0.5 h, and then use a 3500 molecular weight dialysis bag to perform dialysis for 12 h to remove tetrahydrofuran and obtain a dialysate;
[0048] (5) Filter the dialysate with a microporous membrane of 0.22 μm to obtain a clofarabine methotrexate double-drug nanomicelle solution with an average particle diameter of 120 nm;
[0049] (6) The clofarabine and methotrexate double-drug nano-micelle solution was added with sucrose as a freeze-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com