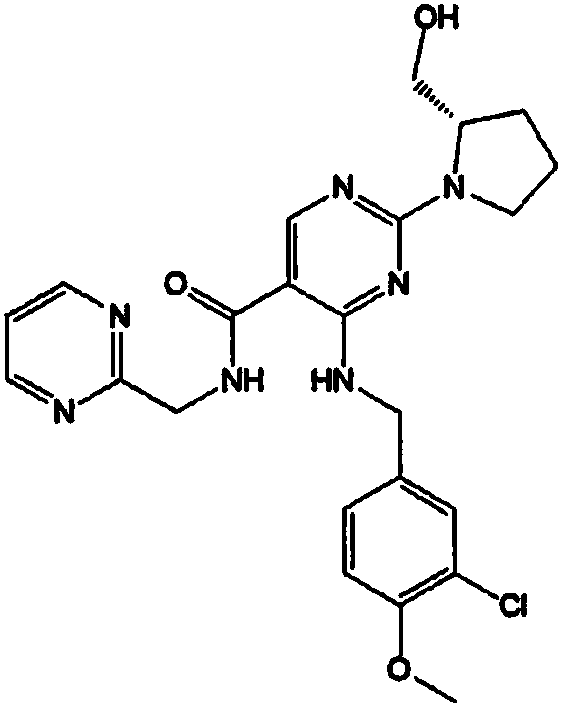
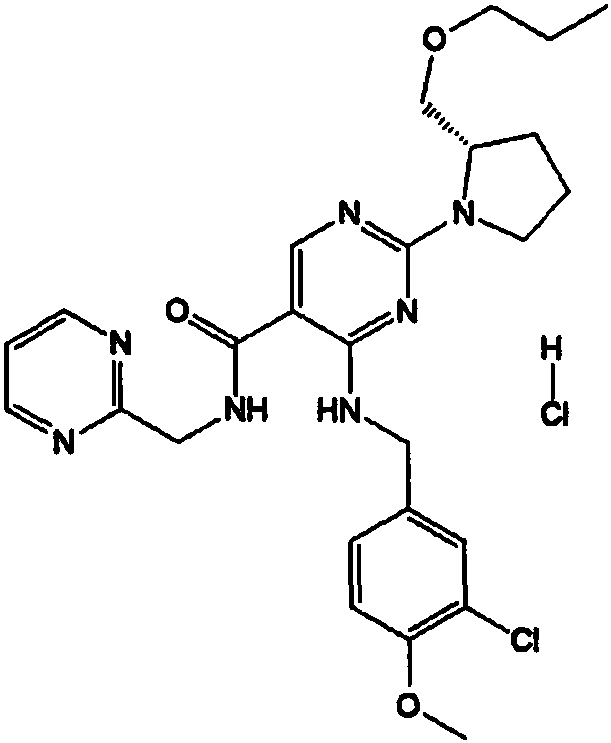
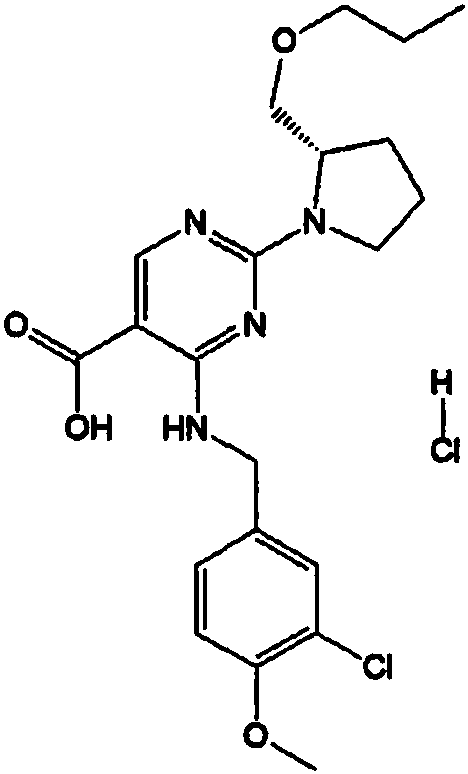
Compound for treating erectile dysfunction of men
A technology of erectile dysfunction and compound, which is applied in the field of medicine, can solve the problems of low incidence of back pain and audio-visual impairment, etc., and achieve the effect of long drug effect time, increased blood flow and remarkable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Randomly select 20 volunteers who suffer from male erectile dysfunction disease, and are divided into two groups on average, with 10 people in each group. The first group of volunteers takes the medicine 100 mg for treating male erectile dysfunction of the present invention, and the second group of volunteers After taking 100 mg of avanafil tablets, the onset time and duration of drug effects of the two drugs were counted, and the physical symptoms of the two groups of volunteers were observed.
[0017] The first group of statistical results shows that the onset time of the medicine of the present invention is 40-120 minutes, and the maintenance time of the drug effect morning erection is about 5-10 days. Adverse reactions;
[0018] The second set of statistics shows that the onset time of avanafil tablets is 20-40 minutes, and the duration of drug effect is 4-6 hours. Adverse reactions such as headache, dizziness, and palpitations may occur, and in severe cases, sudde...
Embodiment 2
[0021] Randomly select 20 volunteers suffering from male erectile dysfunction disease, and divide them into two groups on average, with 10 people in each group. The volunteers in the first group take 100 mg of avanafil tablets first, and take 100 mg of the medicine of the present invention after the efficacy of the drug fails. The second group of volunteers first took 100 mg of the drug of the present invention, and then took 100 mg of avanafil tablets after the efficacy of the drug failed, and then observed the symptoms of the two groups of volunteers in terms of physical fitness.
[0022] Observation results found that after the first group of volunteers took avanafil tablets, adverse reactions such as backache and back pain occurred, and after the efficacy of the drug failed, the above adverse reactions disappeared gradually; the second group of volunteers took the medicine of the present invention Afterwards, no adverse reactions occurred, and after taking avanafil tablets ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com