Organic light-emitting material containing tetraphenylbenzene, preparation and application
A luminescent material, tetraphenylbenzene technology, applied in the preparation of luminescent materials, organic compounds, organic chemistry, etc., can solve the problems of luminescence quenching, difficult luminescent materials, etc., and achieve simple storage, good optoelectronic properties, and stable structure. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
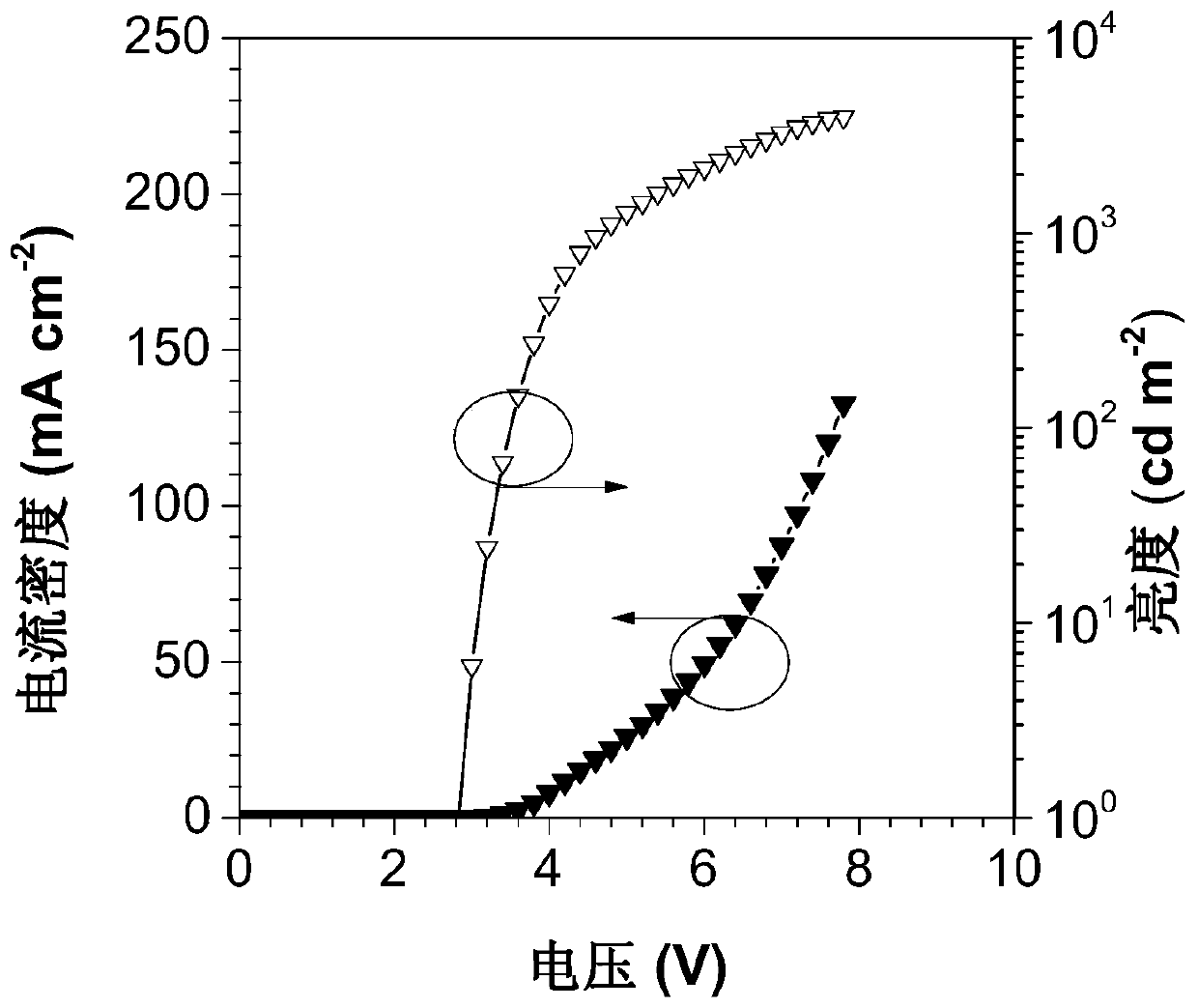
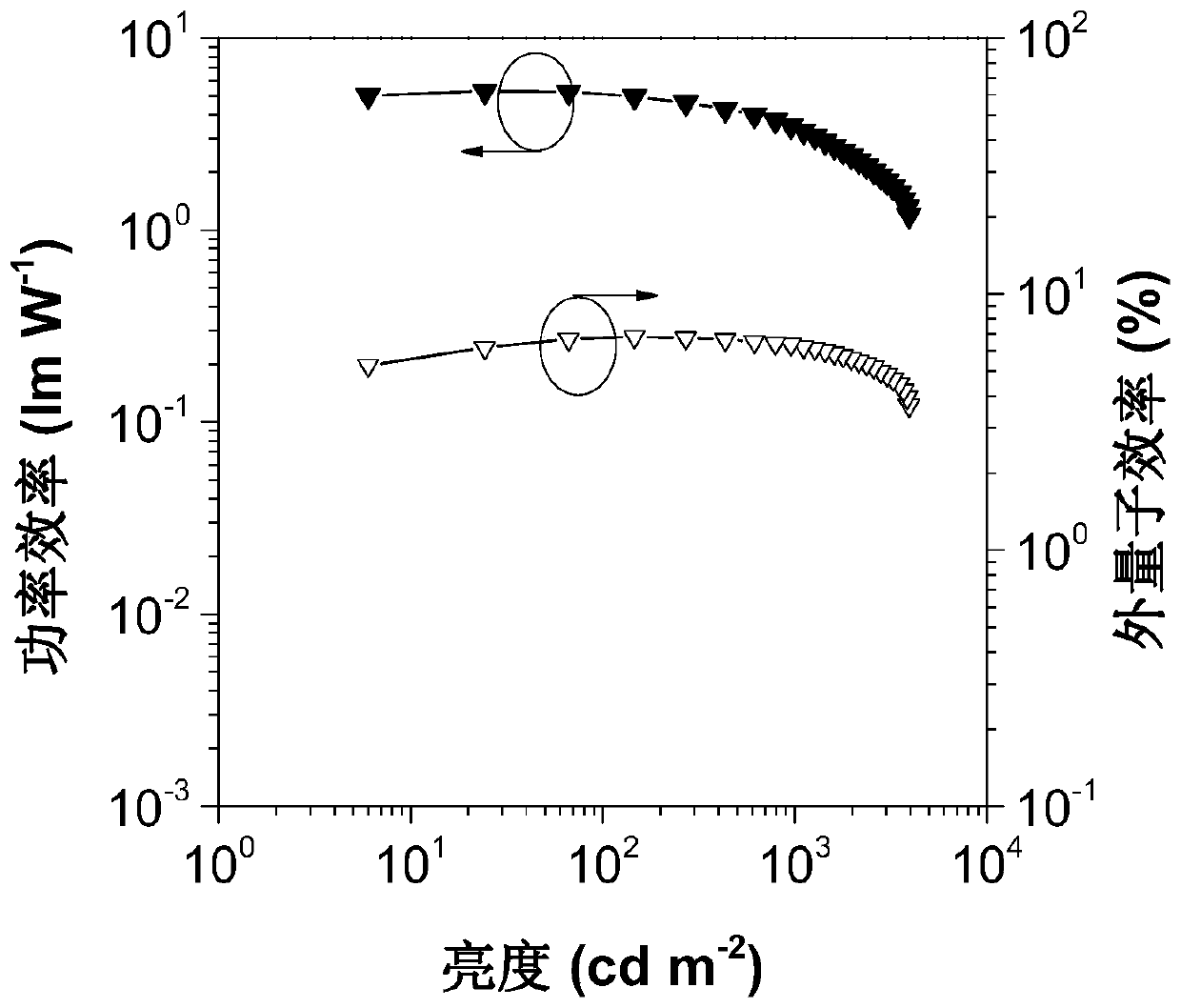
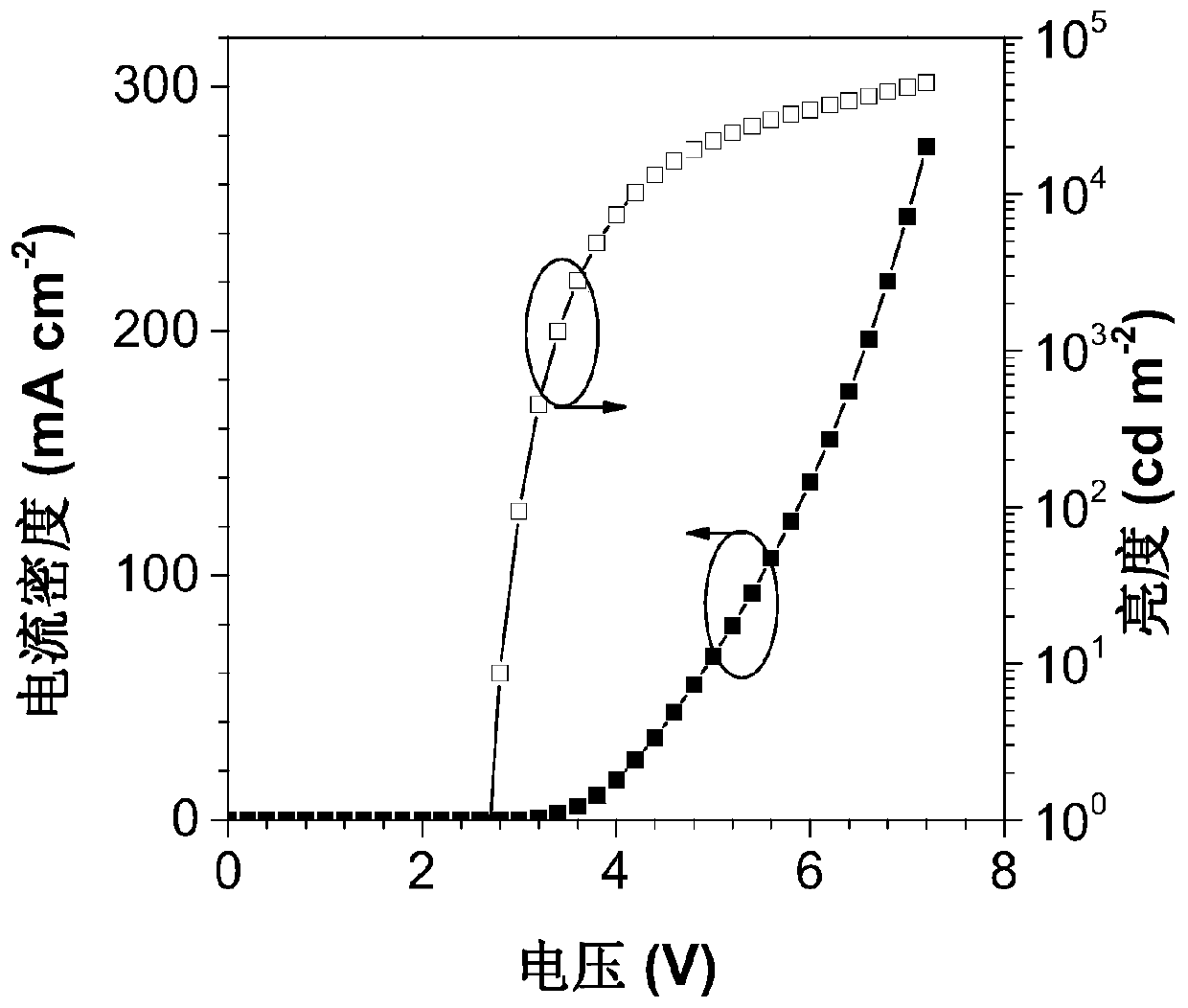
Image
Examples
Embodiment 1
[0034] The preparation of the organic electroluminescent material (TPA-TPB-CN) containing tetraphenylbenzene in this embodiment:
[0035]
[0036] The synthetic route is as follows:
[0037]
[0038] (1) P-bromoterphenyl (compound 1) (6g, 15.5mmol), 4-cyanophenylboronic acid (compound 2) (2.50g, 17.0mmol), anhydrous potassium carbonate (6.4g, 46.5mmol) and Pd (PPh 3 ) 4 (895mg, 0.8mmol) was added to a 250mL reaction flask, under nitrogen protection, 105mL THF and 15mL water were added, refluxed overnight, after the reaction was cooled, it was extracted with dichloromethane, concentrated and then powdered and passed through the column to obtain a white solid 3 with a yield of 73 %;
[0039] (2) Intermediate 3 (2.136g, 1.5mmol), triphenylamine 4-phenylboronic acid (compound 4) (1.6g, 3.9mmol), anhydrous potassium carbonate (1.616g, 11.7mmol) and Pd (PPh 3 ) 4 (225mg, 0.195mmol) was added to a 250mL reaction flask, under the protection of nitrogen, 10mL THF and 5mL wat...
Embodiment 2
[0042] Preparation of the organic electroluminescent material (Cz-TPB-CN) containing tetraphenylbenzene in this embodiment
[0043]
[0044] The synthetic route is as follows:
[0045]
[0046] Intermediate 3 (2.136g, 1.5mmol), 4-boronic acid carbazole (compound 5) (1.119g, 3.9mmol), anhydrous potassium carbonate (1.616g, 11.7mmol) and Pd (PPh 3 ) 4 (225mg, 0.195mmol) was added to a 250mL reaction flask, under the protection of nitrogen, 10mL THF and 5mL water were added, refluxed overnight, the reaction was cooled, extracted with dichloromethane, concentrated, powdered and passed through the column to obtain white solid Cz-TPB-CN , productive rate 86%; Product identification data are as follows:
[0047] 1 H NMR (500MHz, CD 2 Cl 2 ),δ(TMS,ppm):8.16(m,1H),8.14(m,1H),7.69(s,1H),7.58(d,2H),7.56(m,1H),7.47(d,4H) ,7.45-7.38(m,6H),7.35-7.25(m,12H).
Embodiment 3
[0049] Preparation of the organic electroluminescent material (3PhCz-TPB-CN) containing tetraphenylbenzene in this embodiment
[0050]
[0051] The synthetic route is as follows:
[0052]
[0053] Intermediate 3 (2.136g, 1.5mmol), compound 6 (1.415g, 3.9mmol), anhydrous potassium carbonate (1.616g, 11.7mmol) and Pd(PPh 3 ) 4 (225mg, 0.195mmol) was added to a 250mL reaction flask, under the protection of nitrogen, 10mL THF and 5mL water were added, refluxed overnight, after the reaction was cooled, it was extracted with dichloromethane, concentrated, powdered and passed through the column to obtain a white solid 3PhCz-TPB-CN , productive rate 84%; Product identification data are as follows:
[0054] 1 H NMR (500MHz, CDCl 3 ), δ(TMS,ppm):8.35(d,1H),8.18(m,1H),7.59-7.66(m,8H),7.50(d,3H),7.47(m,4H),7.27-7.45( d,13H), 7.29(m,2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| external quantum efficiency | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com