In vivo synthesis of sialylated compounds
A technology of sialylation and sialic acid synthase, which is applied in the fermentation of metabolic engineering microorganisms, synthetic biology and metabolic engineering, can solve the problems of high production cost, loss of productivity and titer, insufficient to meet the large demand of biotechnology, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
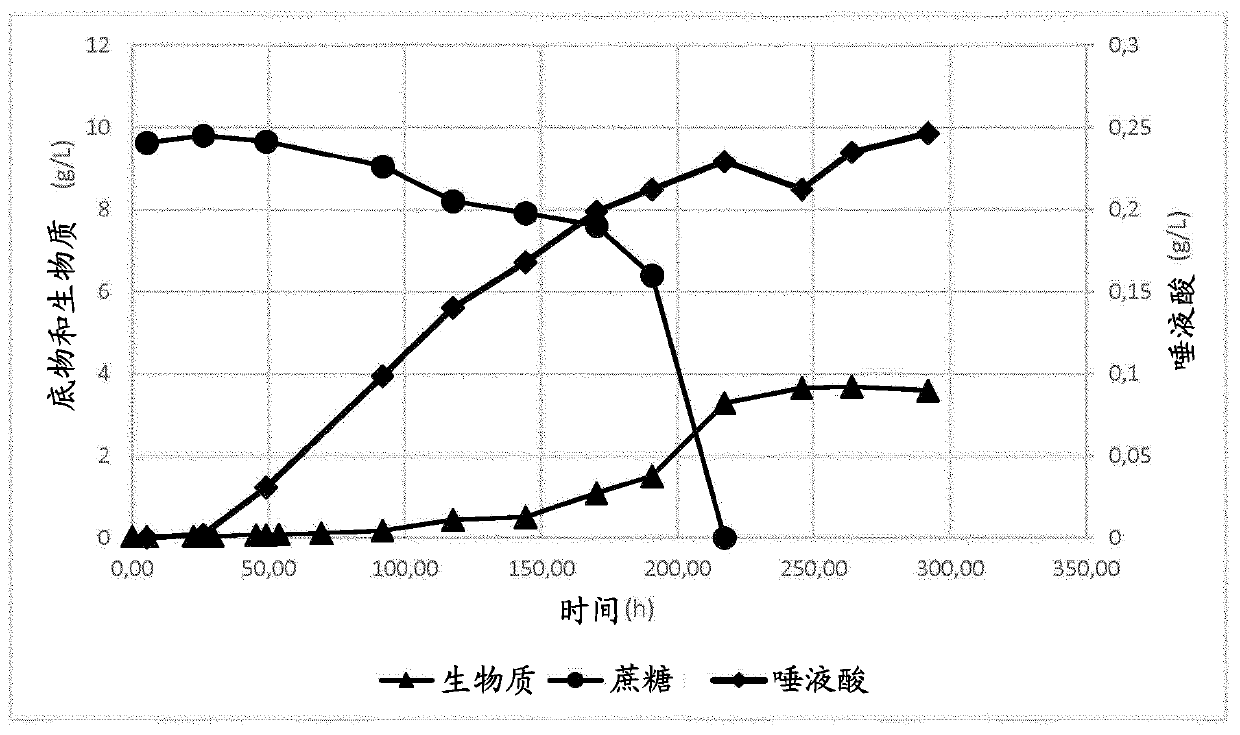
[0021] The present invention describes an economical, more efficient alternative biosynthetic pathway for the production of sialylated compounds using microorganisms.
[0022] The present invention provides methods for producing sialylated compounds by fermentative growth of microorganisms.
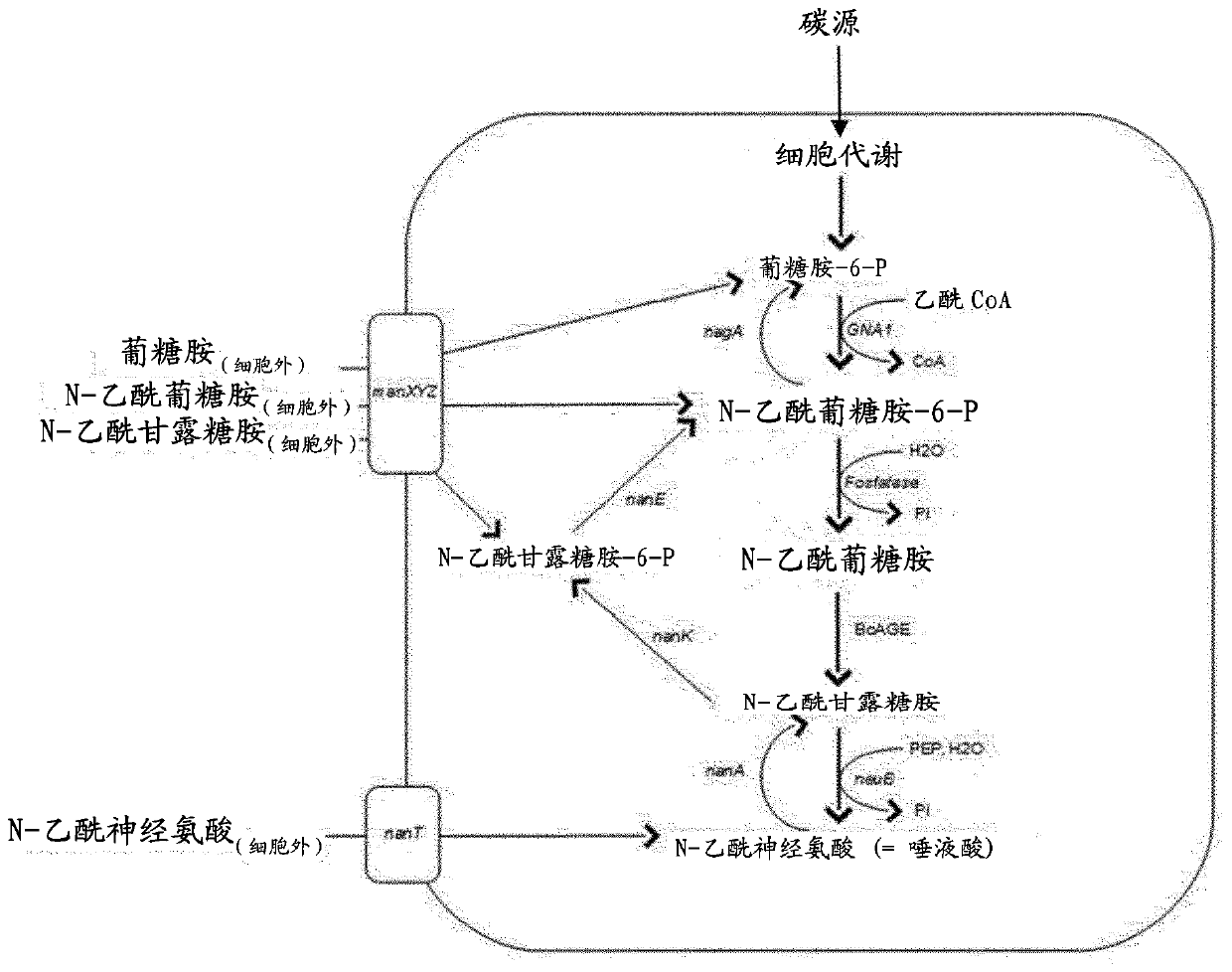
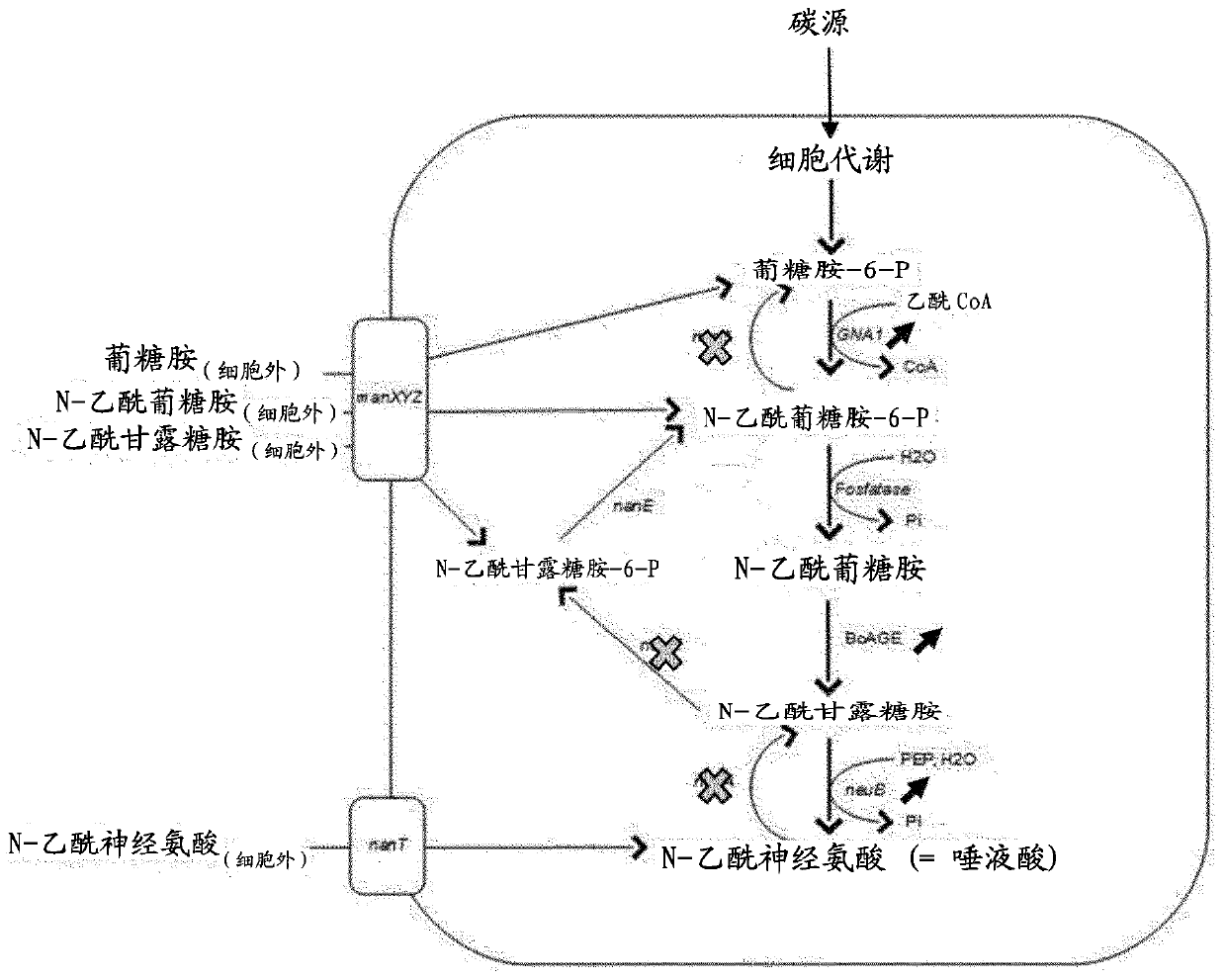
[0023] In particular, the invention relates to a method of producing a sialylated compound, wherein said method comprises culturing a microorganism in a culture medium. Microorganisms convert the following reactions intracellularly: N-acetylglucosamine-6-phosphate to N-acetylglucosamine, N-acetylglucosamine to N-acetylmannosamine, and N-acetylmannosamine It is N-acetylneuraminic acid. In addition, the microorganism is unable to: i) convert N-acetylglucosamine-6-P to glucosamine-6-P, ii) convert N-acetylglucosamine to N-acetylglucosamine-6-P , and iii) converting N-acetylneuraminic acid to N-acetylmannosamine.
[0024] Preferably, the conversion of N-acetylglucosamine-6-phosphate to N-a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com