Cationic liposome containing DHA and HMGB1-silent siRNA, preparation method and application of cationic liposome
A cationic liposome, silent technology, applied in the biological field, can solve the problems of low bioavailability, poor solubility, and single target
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
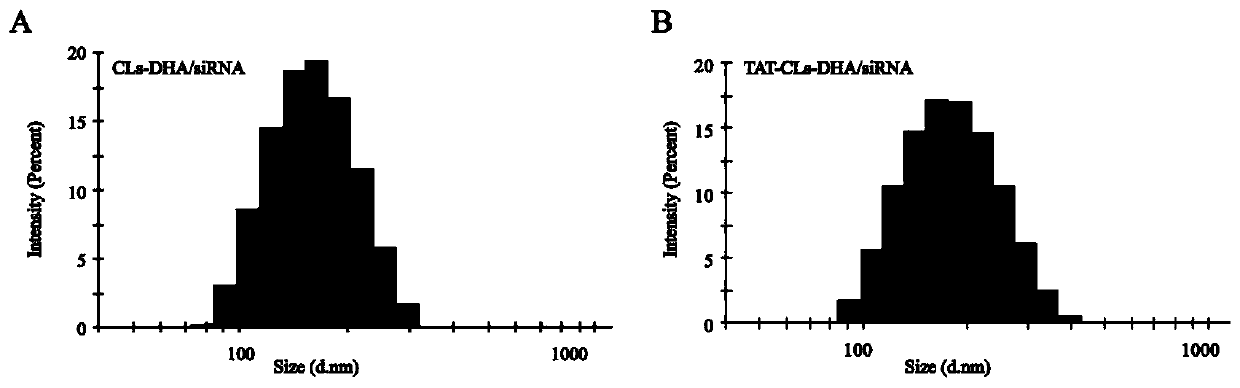
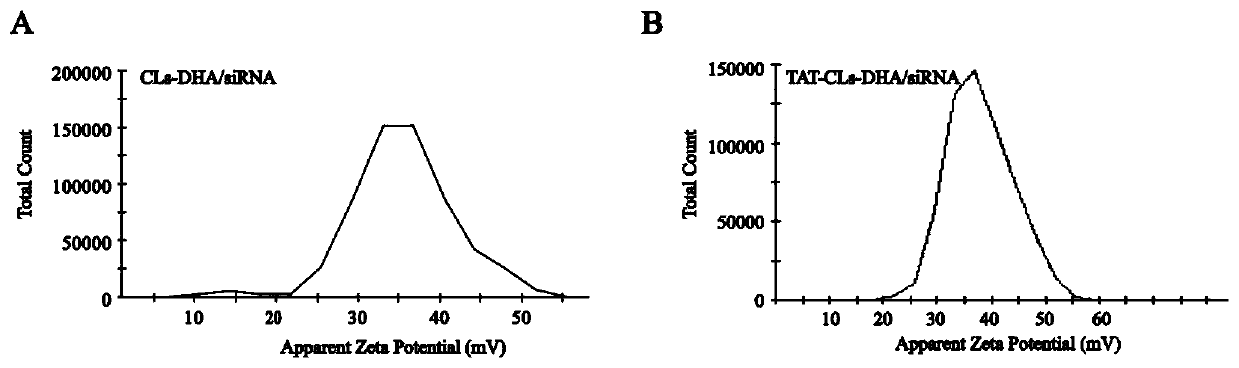
[0066] Embodiment 1, the preparation of the cationic liposome (CLs-DHA / siRNA) of co-carrying DHA and HMGB1 silencing siRNA
[0067] Will DOTAP, Chol, PEG 2000 - DSPE and DHA were dissolved in chloroform according to the molar ratio (2:1:0.15), and the lipid film was formed by rotary evaporation, and a certain amount of PBS was added to hydrate for 30 minutes, so that the final concentration of DHA was 1mg / ml, and then 200nm and 100nm The polycarbonate membrane extruded to obtain the cationic liposome containing DHA with uniform particle size.
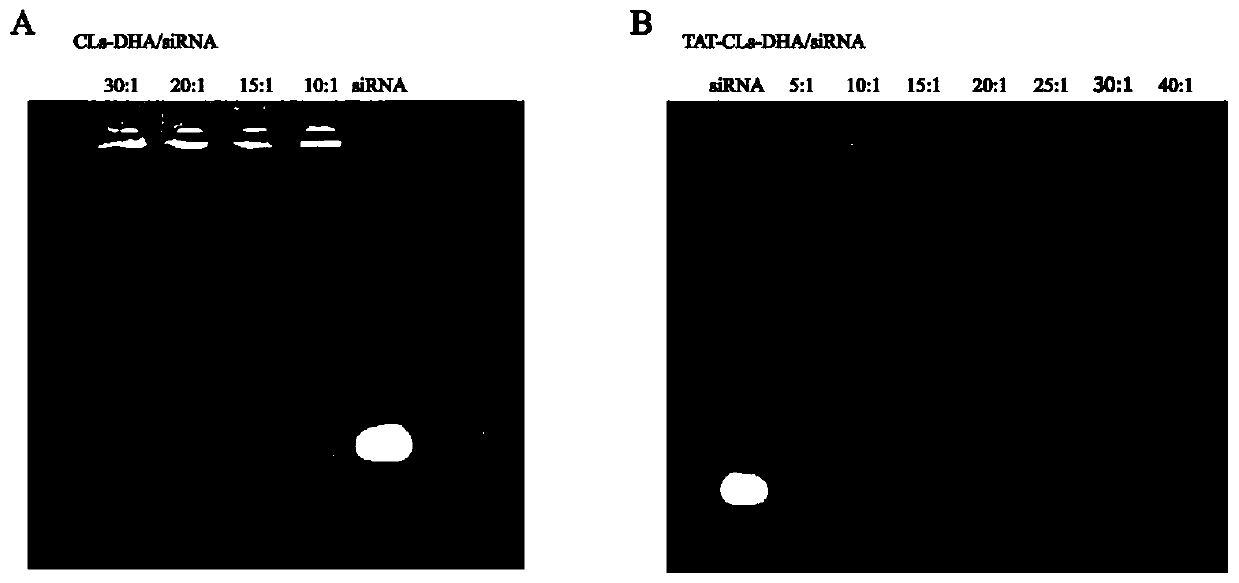
[0068] The cationic liposomes prepared above and HMHGB1 silencing siRNA were mixed according to different mass ratios of DOTAP and siRNA (5:1 / 10:1 / 15:1 / 20:1 / 30:1) and incubated at room temperature for 30 minutes to combine them through electrostatic binding. Cationic liposomes containing DHA and HMGB1-silencing siRNA were prepared by binding HMGB1-silencing siRNA to liposomes. The ratio of DOTAP to HMGB1 silencing siRNA was examined b...
Embodiment 2
[0070] Example 2, preparation of TAT peptide (AYGRKKRRQRRR) modified cationic liposomes (TAT-CLs-DHA / siRNA) loaded with DHA and HMGB1 silencing siRNA
[0071] 1 TAT-mPEG 2000 -DSPE synthesis
[0072] One-step synthesis of TAT-mPEG 2000 -DSPE. We added a cysteine (cys) to the N-terminus of the TAT peptide to introduce a sulfhydryl group for compatibility with DSPE-PEG 2000 The maleimide at the -Mal terminal undergoes direct addition reaction. Specifically, TAT and DSPE-PEG 2000 -Mal was dissolved in PBS at a molar ratio of 1:1, under nitrogen protection, at 4°C, and reacted overnight to prepare TAT-mPEG 2000 -DSPE.
[0073] 2 Preparation of TAT peptide-modified cationic liposomes co-loaded with DHA and HMGB1 silencing siRNA
[0074] DOTAP, Chol, the above TAT-mPEG 2000 -DSPE and DHA were dissolved in chloroform according to the molar ratio of 2:1:0.15:0.33, and the lipid film was formed by rotary evaporation, and a certain amount of PBS was added to hydrate for 30 min...
Embodiment 3
[0077] Embodiment 3, the cationic liposome (CLs-DHA / siRNA) of co-loading DHA and HMGB1 silencing siRNA and the TAT peptide modified cationic liposome (TAT-CLs-DHA / siRNA) of co-loading DHA and HMGB1 silencing siRNA ) Stability study
[0078] Prepare the complex of the optimal prescription (DOTAP / siRNA mass ratio is 15:1), mix it with 20% fetal bovine serum at 1:1 (volume ratio), so that the final serum concentration is 10%, and place the sample in a 37°C water bath Incubation. After incubation for a certain period of time, an appropriate amount of Triton-100 solution was added to the sample to extract siRNA, and immediately mixed with 6x Loading Buffer, analyzed by agarose gel electrophoresis and photographed by a gel imaging system.
[0079] For cationic liposomes co-loaded with DHA and siRNA, by Figure 5 A shows that the siRNA encapsulated in the liposome remains stable within 36h in serum and is gradually degraded after 36h; while naked siRNA is generally degraded in 6h i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Electric potential | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com