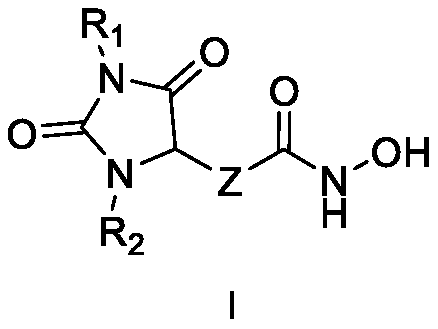
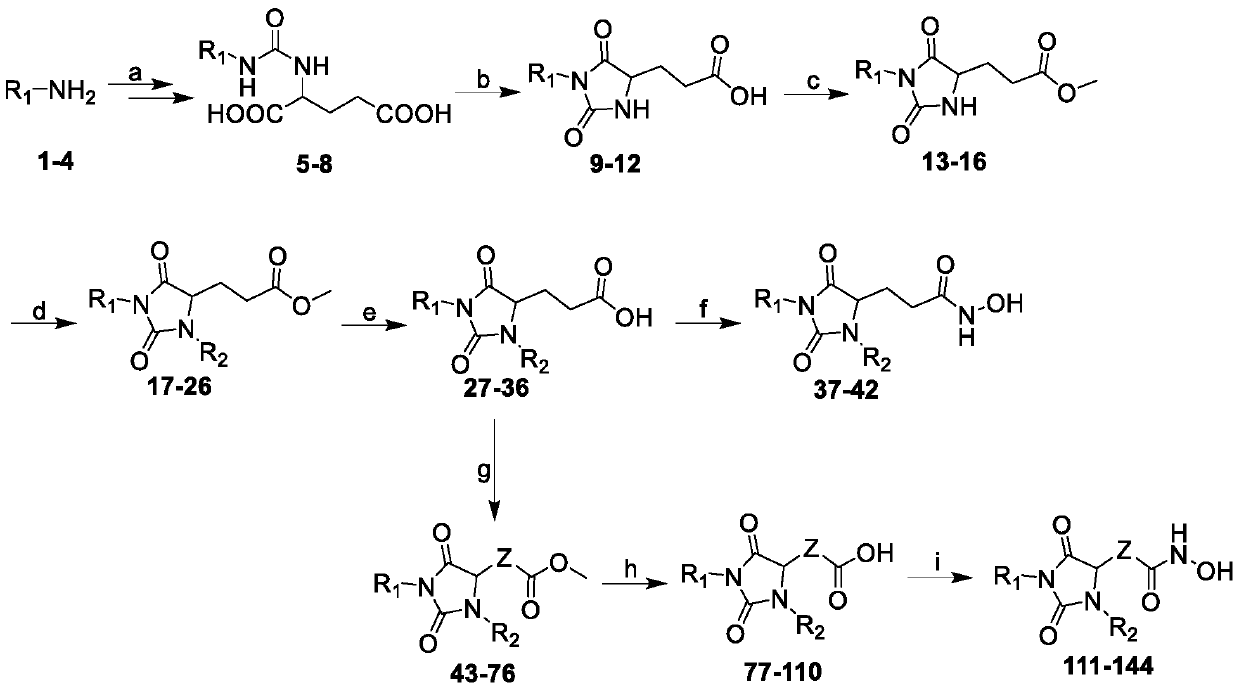
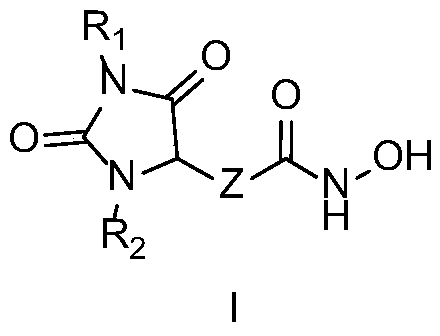
Hydantoin hydroxamic acid type selective inhibitor for histone deacetylase 6 subtype and preparation method and applications thereof
A technology of hydantoin hydroxime and sirtuin, which is applied in the field of medicine and can solve the problems of limiting the clinical application of HDAC inhibitors, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] Example 1. Synthesis of ((4-chlorophenyl) carbamoyl) glutamic acid (5)
[0133] Dissolve p-chloroaniline (10.2g, 80mmol) in dichloromethane (150mL), slowly drop it into a solution of triphosgene (8.01g, 27mmol) in dichloromethane under ice-cooling; after the addition is complete, react under ice-cooling 30min. with saturated NaHCO 3 Quench the reaction with solution, extract the organic phase, anhydrous MgSO 4 After drying, the solvent was evaporated under reduced pressure to obtain p-chlorophenyl isocyanate. Dissolve L-glutamic acid (11.77g, 80mmol) with 2M NaOH (150mL) solution, dissolve the newly prepared chlorophenylisocyanate with toluene, then slowly drop it into the glutamic acid solution under ice-bath conditions, and Reaction 6h. Separate the aqueous phase, adjust the pH to 2 with 6M hydrochloric acid, then extract three times with ethyl acetate, combine the organic phases, anhydrous MgSO 4 Drying, column chromatography gave white solid 14.48g, yield 68%, ...
Embodiment 2
[0149] Example 2. 3-(1-(4-chlorophenyl)-3-(4-methylbenzyl)-2,5-dioximidazolin-4-yl)-N-hydroxypropionamide (38)
[0150] The preparation method of intermediate and target object is as embodiment 1, yield: 58%, melting point: 135-138 ℃. 1 H NMR (400MHz, CDCl 3 )δ8.91(s,1H),8.32(s,1H),7.38(d,J=8.8Hz,2H),7.34(d,J=8.9Hz,2H),7.18(d,J=8.0Hz, 2H), 7.15(d, J=8.0Hz, 2H), 4.92(d, J=15.1Hz, 1H), 4.14(d, J=15.1Hz, 1H), 3.93(d, J=3.5Hz, 1H ),2.33(s,3H),2.32–2.24(m,1H),2.16(d,J=7.2Hz,2H),2.06(dd,J=13.4,6.3Hz,1H). 13 C NMR (151MHz, DMSO-d 6 )δ171.73, 168.28, 155.32, 137.22, 133.69, 132.77, 131.42, 129.70, 129.23, 128.90, 128.38, 58.70, 44.49, 26.72, 24.48, 21.19. HRMS (AP-ESI) m / z, Calcd for C 20 h 21 ClN 3 o 4 ,([M+H] + ):402.1215,found:402.1212.
Embodiment 3
[0151] Example 3. 3-(3-Benzyl-1-(4-chlorophenyl)-2,5-dioximidazolin-4-yl)-N-hydroxypropionamide (39)
[0152] The preparation method of intermediate and target object is as embodiment 1, yield: 83%, melting point: 75-78 ℃. 1 H NMR (400MHz, DMSO-d 6 )δ10.41(s,1H),8.75(s,1H),7.58(d,J=8.7Hz,2H),7.48(d,J=8.7Hz,2H),7.44–7.26(m,5H). ,4.75(d,J=15.8Hz,1H),4.39(d,J=15.8Hz,1H),4.20(t,J=4.1Hz,1H),2.18–1.86(m,4H). 13 C NMR (151MHz, DMSO-d 6 )δ171.75, 168.27, 155.40, 136.82, 132.78, 131.42, 129.24, 129.12, 128.92, 128.32, 128.03, 58.88, 44.78, 26.71, 24.52. HRMS (AP-ESI) m / z, Calcd for C 19 h 19 ClN 3 o 4 ,([M+H] + ):388.1059,found:388.1058.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com