Dual-core metal organic complex with asymmetric structure and preparation and applications thereof
An organic complex, binuclear metal technology, applied in structural asymmetric binuclear metal-organic complex and its preparation, metal-organic complex, metal-organic complex as a catalyst in the application field, can solve problems such as complex synthesis, achieve high reaction Active, extended industrial application range, wide-ranging effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The specific reaction process of the metal organic complex I-1 is represented by the following reaction equation.
[0053]
[0054] In the absence of water and oxygen, 0.05mol 2,2'-dihydroxy-[1,1'-diphenyl]-3-carbaldehyde (synthesized by the method of document Organometallics, 2007, 26, 2609‒2615), 0.075mol Polyoxymethylene and 0.05mol morpholine were dissolved in toluene, heated to reflux for 5h. Extract with ethyl acetate, wash with sodium chloride solution, dry over sodium sulfate, and evaporate the solvent by rotary evaporation. The obtained solid was dissolved in a mixed solvent of ethyl acetate and petroleum ether, the target compound solution was separated by a chromatographic column, and the solvent was rotary evaporated to obtain a white solid 2,2'-dihydroxy-3'-(morpholine-4- Base)-[1,1'-diphenyl]-3-carbaldehyde 10.5g, yield 67%.
[0055] In the absence of water and oxygen, mix 0.02mol 2,2'-dihydroxy-3'-(morpholin-4-yl)-[1,1'-diphenyl]-3-carbaldehyde with ...
Embodiment 2
[0062] The specific reaction process of the metal organic complex I-2 is represented by the following reaction equation.
[0063]
[0064] In the absence of water and oxygen, dissolve 0.05mol 2,2'-dihydroxy-[1,1'-diphenyl]-3-carbaldehyde, 0.075mol polyoxymethylene and 0.05mol pyrrolidine in toluene, and heat to reflux for reaction 7h. Extract with ethyl acetate, wash with sodium chloride solution, dry over sodium sulfate, and evaporate the solvent by rotary evaporation. The obtained solid was dissolved in a mixed solvent of ethyl acetate and petroleum ether, the solution of the target compound was separated by a chromatographic column, and the solvent was rotary evaporated to obtain a white solid 2,2'-dihydroxy-3'-(pyrrolidine-4- Base)-[1,1'-diphenyl]-3-carbaldehyde 9.3g, yield 63%.
[0065] In the absence of water and oxygen, 0.03mol 2,2'-dihydroxy-3'-(pyrrolidin-4-yl)-[1,1'-diphenyl]-3-carbaldehyde and 0.03mol 1-benzyl Basepiperidin-4-amine was heated under reflux in m...
Embodiment 3
[0070] The specific reaction process of the metal organic complex I-3 is represented by the following reaction equation.
[0071]
[0072] In the absence of water and oxygen, take 0.004mol of the ligand L1 prepared in Example 1 and dissolve it in 20ml of toluene, and slowly add 0.008mol of the metal alkyl compound In(CH 2 SiMe 3 ) 3 The toluene solution was heated to 100°C for 5 h, and the reaction solvent was removed under vacuum to obtain 3.8 g of metal organic complex I-3 light yellow powder with a yield of 89%.
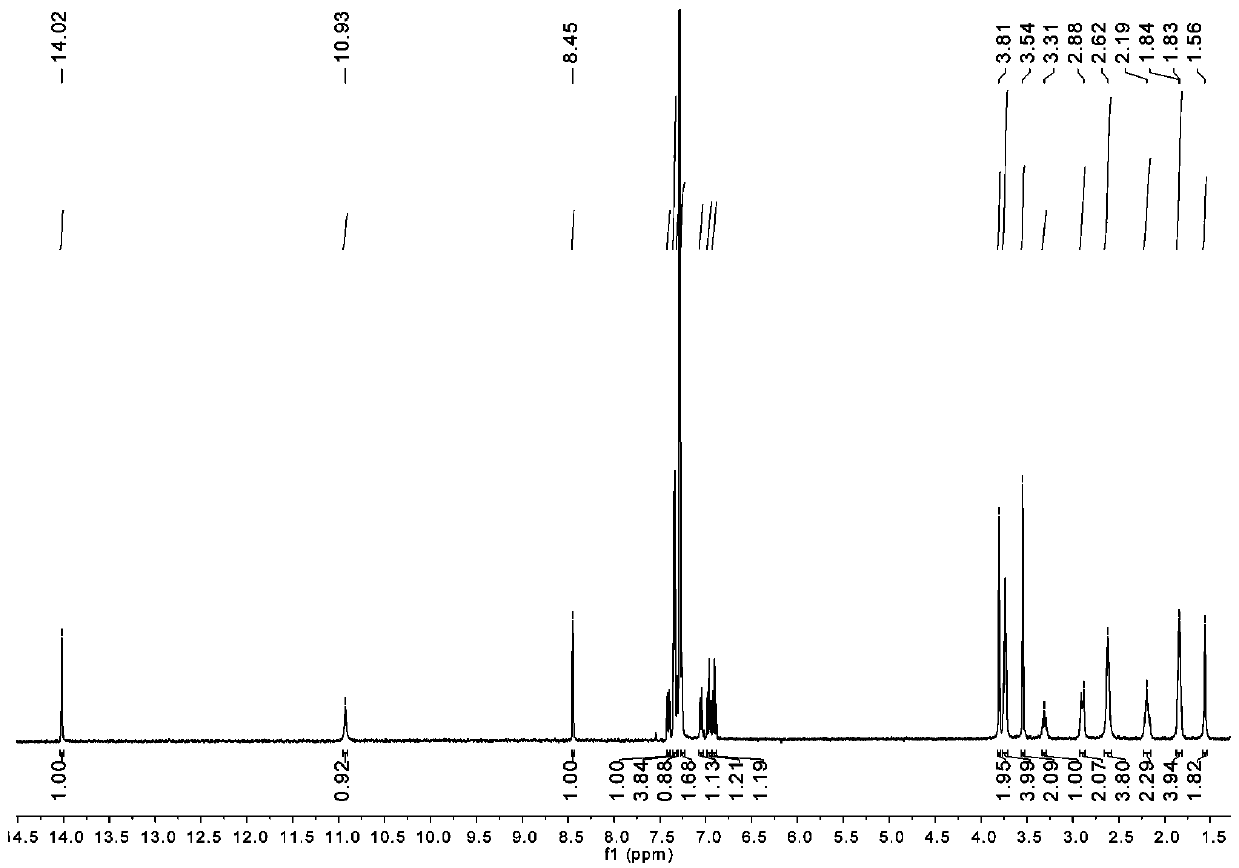
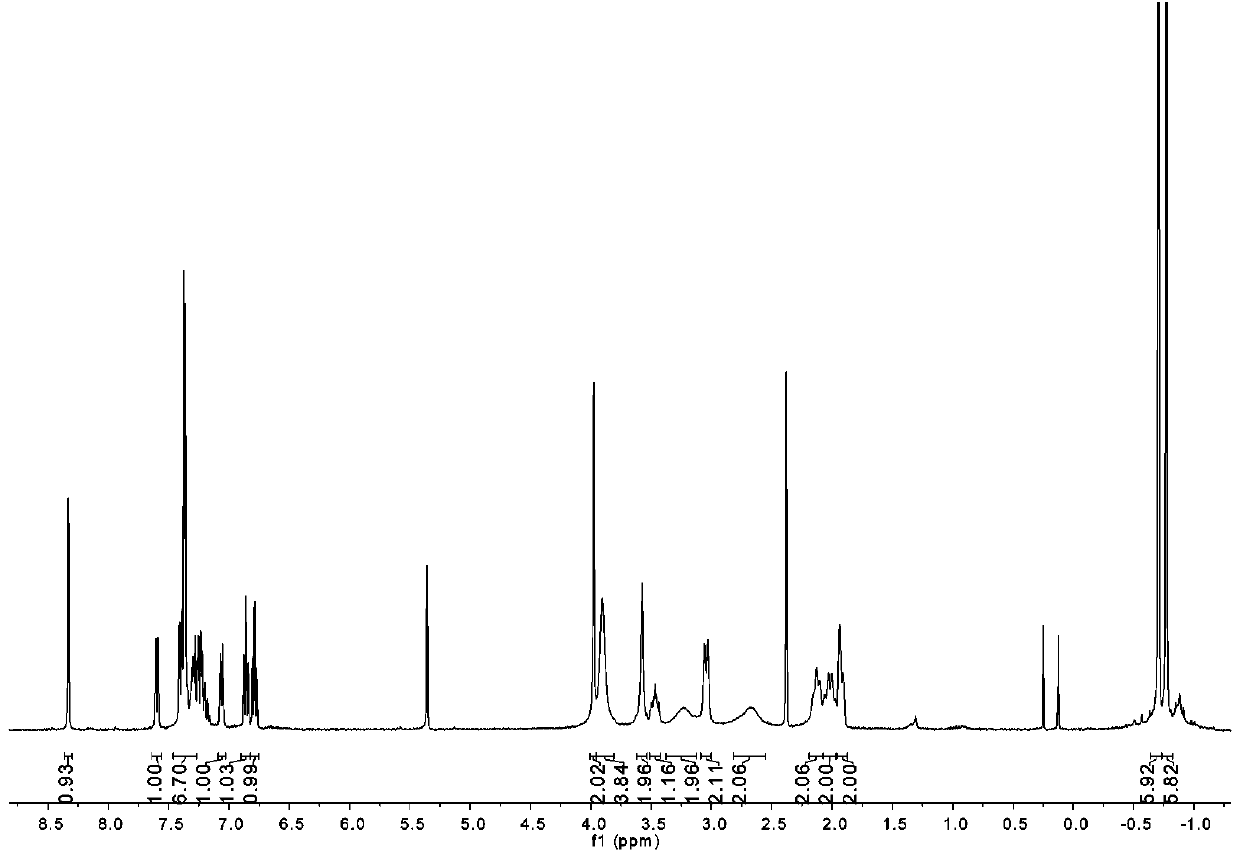
[0073] 1 H-NMR (298 K, CD 2 Cl 2 , 400 MHz): δ 8.31 (d, J = 4.4 Hz, 1H,C H N), 7.61(dd, J = 7.4, 1.8 Hz, 1H, Ar- H ), 7.47 – 7.32 (m, 7H, Ar- H ), 7.26 (dd, J =7.4, 1.7 Hz, 1H, Ar- H ), 6.86 (t, J = 7.6 Hz, 1H, Ar- H ), 6.79 (t, J = 7.5 Hz,1H, Ar- H ), 3.98 (s, 2H, ArC H 2 N), 3.89 (d, J = 18.6 Hz, 4H, morpholin-C H 2 ),3.57 (s, 2H, ArC H 2 N pip), 3.50 – 3.43 (m, 1H, C H pip), 3.21 (s, 2H, morpholin-C H 2 ), 3.08 – 3.00 (m, 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com