Engineered immune cell targeting CD19 and CD22 and application of engineered immune cell
A targeted, cellular technology, applied in the field of immunotherapy, can solve the problems of high recurrence rate and low safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0320] Structural design and virus preparation of embodiment 1CAR
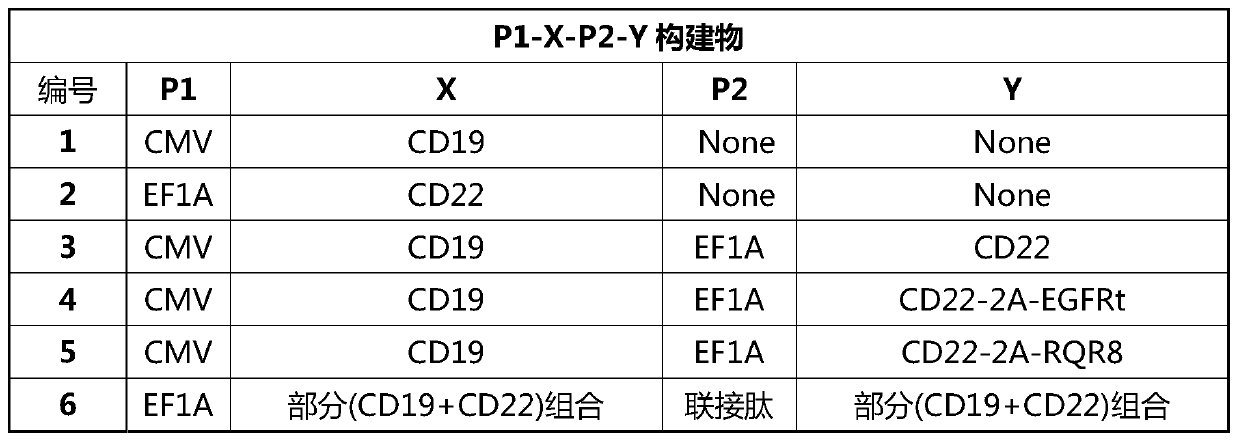
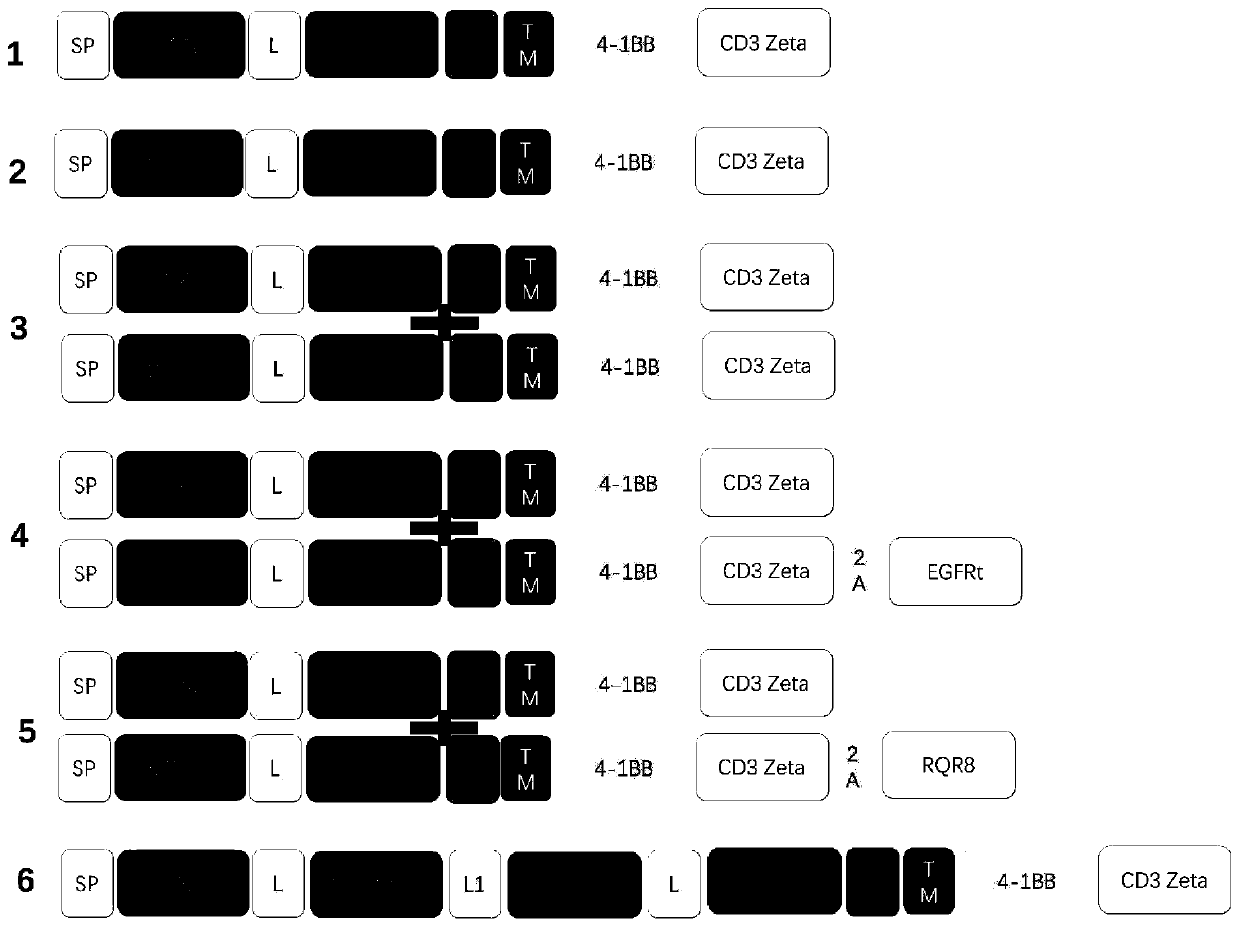
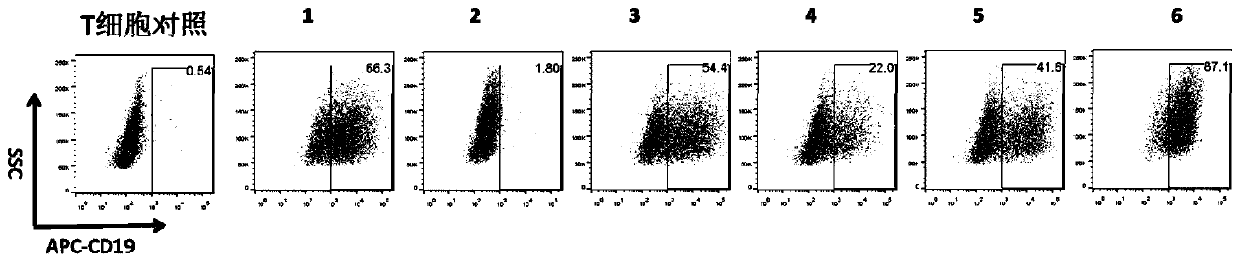
[0321] The present invention provides a nucleic acid construct that co-expresses a specific chimeric antigen receptor for the CD19 antigen and a specific chimeric antigen receptor for the CD22 antigen. The co-expression of a CD19-targeting CAR and a CD22-targeting CAR structural component may include a CD19-targeting CAR and a CD22-targeting CAR.
[0322] The structure of each construct is as Figure 1A with 1B shown.
[0323] Construct a lentiviral transfer vector plasmid that has co-expressed CD19 and CD22. The backbone of the plasmid is derived from pCDH; the lentiviral transfer vector plasmid pCDH-EF1α-CD19 / CD22CAR, and the lentiviral envelope plasmid pMD2.G (Addgene, Plasmid#12259 ) and the lentiviral packaging plasmid psPAX2 (Addgene, Plasmid#12260) were simultaneously transferred into 293T using Lipofectamine3000 to prepare the lentiviral expression vector; the virus supernatant was collected on the s...
Embodiment 2
[0324] Example 2 Isolation and expansion of T cells
[0325] Mononuclear cells were isolated from peripheral blood by density gradient centrifugation using Histopaque-1077 (Sigma-Aldrich), and mononuclear cells were isolated from peripheral blood and enriched for T cells (EasySep human T cell enrichment kit, Stemcell Technologies) Anti-CD3 / anti-CD28 magnetic beads were used to activate, culture and expand T cells; the culture medium used X-vivo15 (containing 5% FBS, 2mM L-glutamine, 1mM sodium pyruvate, 300IU / ml rhIL2); all cells were Place at 37°C, 5% CO 2 cultured in a constant temperature incubator.
Embodiment 3
[0326] Example 3 Cell Culture
[0327] Cell lines expressing CD19 / CD22: Raji (Burkitt”s lymphoma cell line, ATCC-CCL86) Raji-Luc cell line obtained after screening monoclonal infection of Raji cells with firefly luciferase lentivirus; Hela cell line (human cervical cancer cell line Line, ATCC-CCL2), use CD19, CD22 lentivirus transfection to construct stable transfection strains Hela-CD19 and Hela-CD22, use CD22 to infect Hela-CD19 cell line to obtain double-expressed cell line Hela-CD19 / CD22 cells above all Use RPMI 1640 medium to cultivate; 293T (ATCC-CRL3216) to use DMEM medium to cultivate.All mediums are supplemented with 10% (v / v) bovine serum and 100U / ml of penicillin and streptomycin, 2mM glutamine, 1mM Sodium pyruvate; all cells were kept at 37°C, 5% CO 2 cultured in a constant temperature incubator.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com