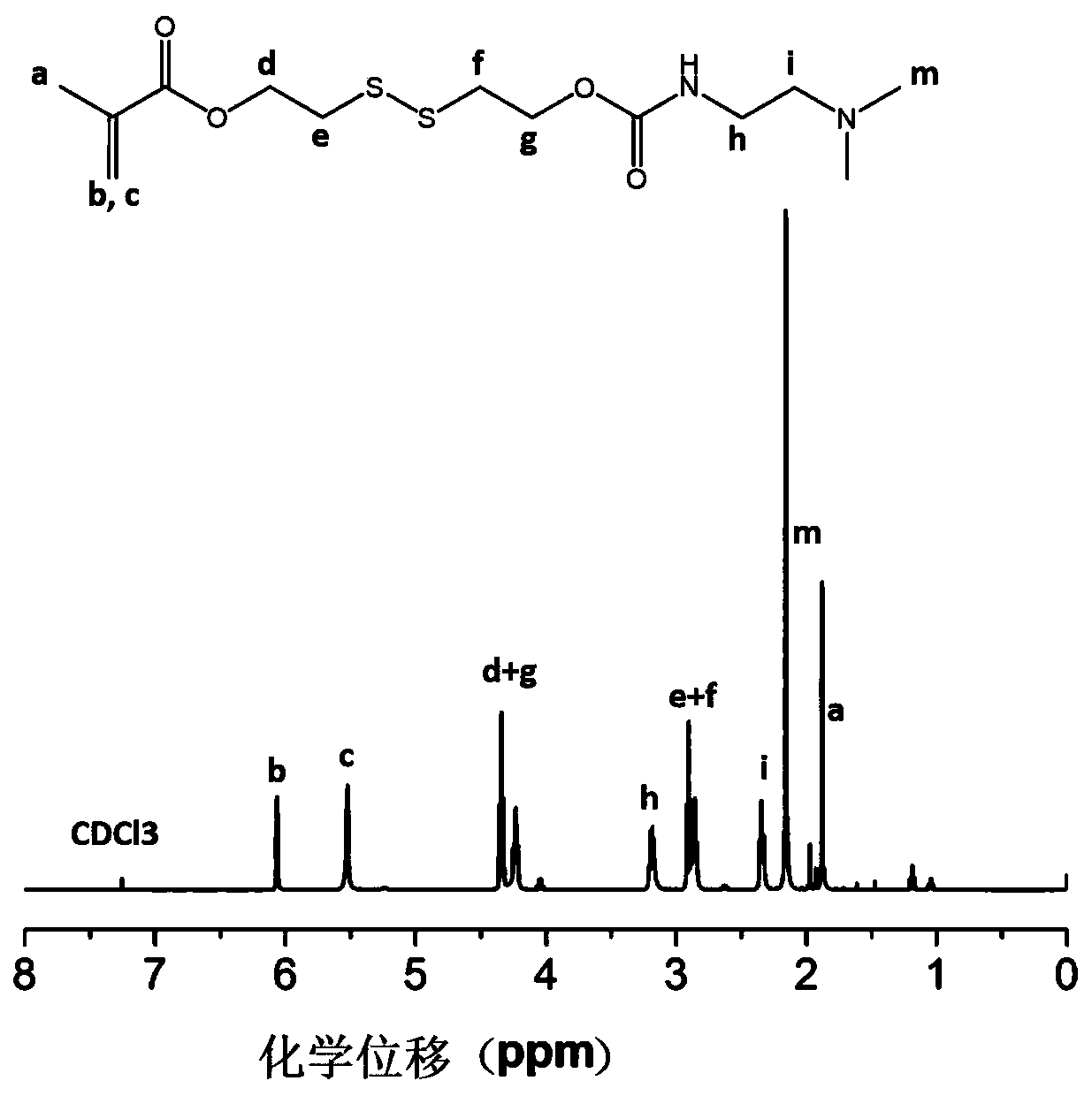
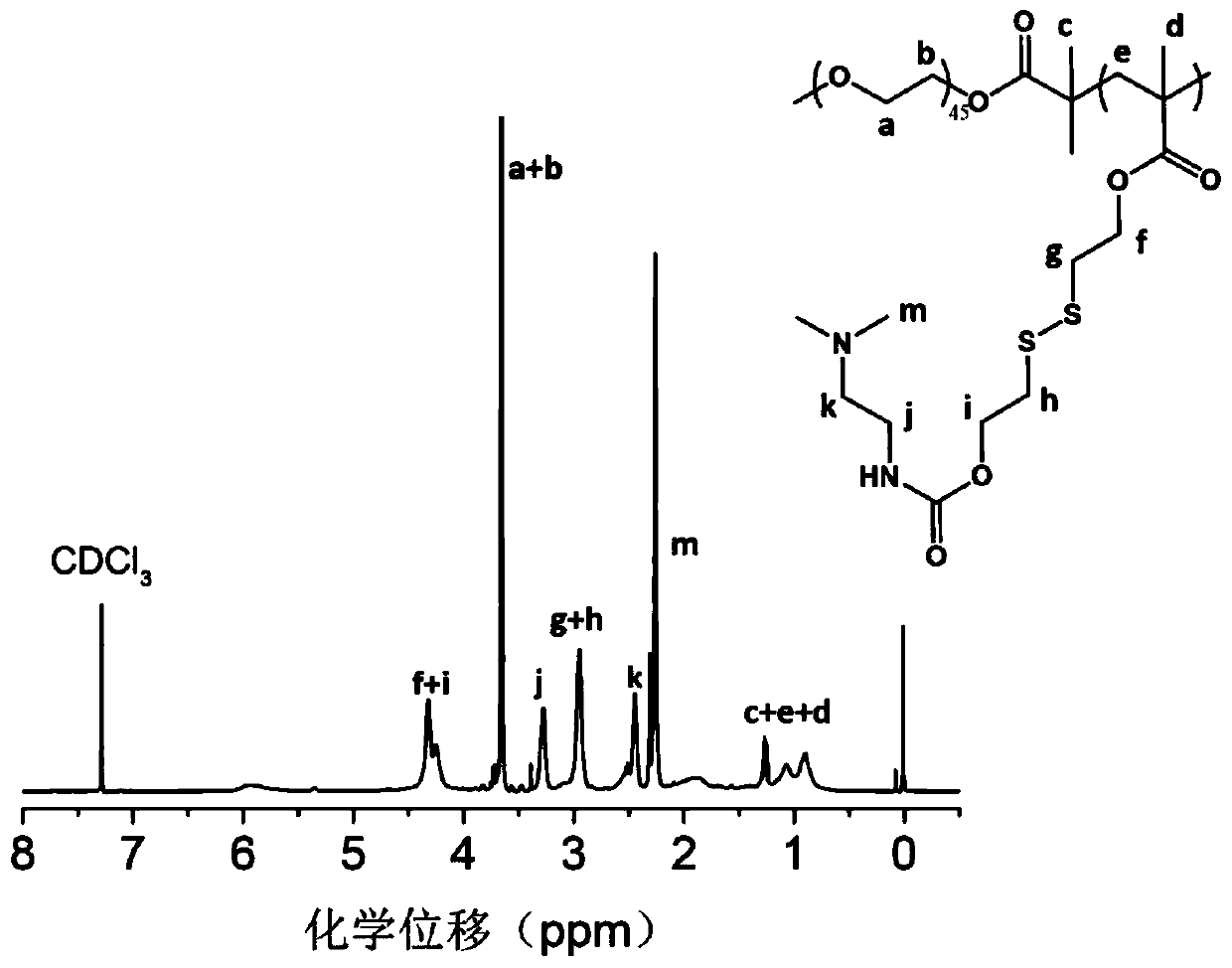
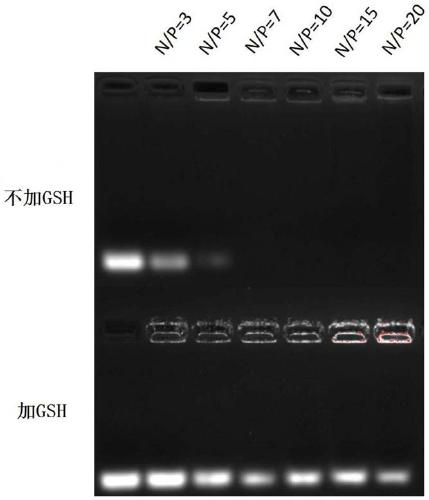
Methacrylic acid-3,4-dithio-hydrohexylacrylate derivative as well as preparation method and application thereof
A technology of dithiohydroxyhexyl ester and methacrylic acid, applied in the field of methacrylic acid-1-hexanol) ester and preparation thereof, can solve the problems of inconvenient operation, complicated technical requirements for PDMAEMA modification and the like, and achieves improved transfection efficiency. effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 30mmol of 3,4-dithiohydroxyhexyl methacrylate, 30mmol of p-nitrochloroformate and 30mmol of triethylamine into the reactor, dissolve with tetrahydrofuran, and react for 12h at room temperature to terminate the reaction. Filter with NaHCO 3 The solution and saturated NaCl solution were washed, and the crude product was obtained as a light yellow oily liquid after rotary evaporation. Purified by silica gel column, the intermediate product 24mmol methacrylic acid (3,4-dithio-6-(p-nitrobenzylcarbonic acid) Ester)-1-hexanol) ester;
[0025] Add 20mmol methacrylic acid (3,4-dithio-6-(p-nitrobenzyl carbonate)-1-hexanol) ester and 20mmol N,N-dimethylaminoethylenediamine into the reactor, Dissolve in tetrahydrofuran, react at room temperature for 24h, stop the reaction, use NaHCO 3 The solution and saturated NaCl solution were washed, and the crude product was obtained as a yellow oily liquid after rotary evaporation. Purification by silica gel column was used to obtain 14mmol ...
Embodiment 2
[0027] Add 20mmol of 3,4-dithiohydroxyhexyl methacrylate, 30mmol of p-nitrochloroformate and 30mmol of triethylamine into the reactor, dissolve it with tetrahydrofuran, and react at room temperature for 6 hours to terminate the reaction. Filter with NaHCO 3 The solution was washed with saturated NaCl solution, and the crude product was obtained after rotary evaporation. The crude product was purified by silica gel chromatography to obtain the intermediate product 18mmol methacrylic acid (3,4-dithio-6-(p-nitrobenzyl carbonate). Ester)-1-hexanol) ester;
[0028] Add 15mmol methacrylic acid (3,4-dithio-6-(p-nitrobenzylcarbonate)-1-hexanol) ester and 20mmol N,N-dimethylaminoethylenediamine into the reactor, Dissolve with tetrahydrofuran, react at room temperature for 6h, stop the reaction, use NaHCO 3 The solution was washed with saturated NaCl solution, and the crude product was obtained as a yellow oily liquid after rotary evaporation. Purification by silica gel column was used to ...
Embodiment 3
[0030] Add 15mmol of 3,4-dithiohydroxyhexyl methacrylate, 20mmol of p-nitrochloroformate and 30mmol of triethylamine into the reactor, dissolve with 150mL of tetrahydrofuran, and react at room temperature for 12h to terminate the reaction , Filter, use NaHCO 3 The solution and saturated NaCl solution were washed, and the crude product was obtained as a light yellow oily liquid after rotary evaporation. The intermediate product 14mmol methacrylic acid (3,4-dithio-6-(p-nitrobenzyl carbonate) was purified by silica gel chromatography. Ester)-1-hexanol) ester;
[0031] Add 10mmol methacrylic acid (3,4-dithio-6-(p-nitrobenzyl carbonate)-1-hexanol) ester and 20mmol N,N-dimethylaminoethylenediamine into the reactor, Dissolve with 100mL tetrahydrofuran, react at room temperature for 12h, stop the reaction, use NaHCO 3 The solution and saturated NaCl solution were washed, and the crude product was obtained as a yellow oily liquid after rotary evaporation, which was purified by a silica ge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com