Methacrylic acid orthoesters new monomer, as well as preparation method and application of amphiphilic block copolymer of methacrylic acid orthoesters new monomer
A methacrylic acid ortho-acid and amphiphilic block technology, which can be used in pharmaceutical formulations, medical preparations of non-active ingredients, organic chemistry, etc. It can solve the problems of difficult removal of metal compounds, inconvenient handling, wide distribution of polymers, etc. problems, to achieve good application prospects, good acid sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
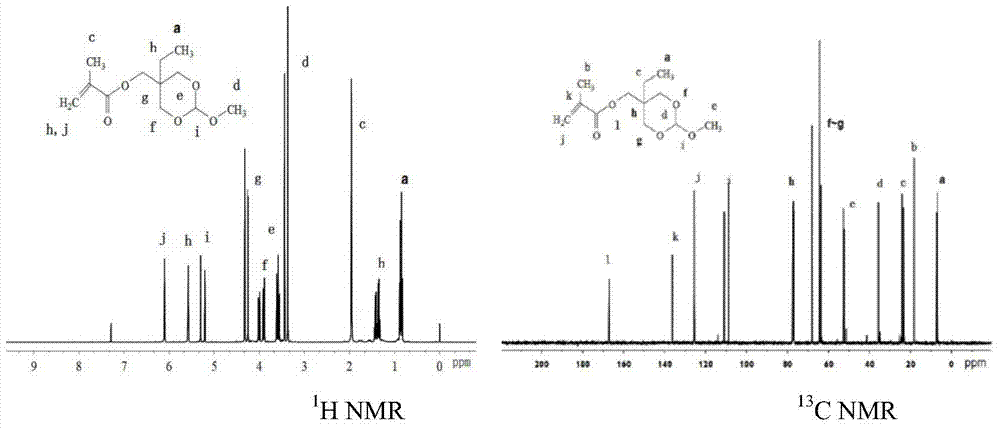
[0035] The synthetic method of 2-methoxy-5-ethyl-[1,3]-dioxane-5-acetoacetate methyl ester monomer is realized through the following steps:
[0036] 1) Preparation of ethyl 2,2-dimethylolbutyrate
[0037] Under nitrogen atmosphere, add 20.00g (135.41mmoL) 2,2-dihydroxybutyric acid, 120mL absolute ethanol, 2mL concentrated sulfuric acid (98%) into a 250ml three-neck flask, reflux at 130°C for 12 hours, cool at room temperature, add Excess sodium carbonate solid neutralizes the remaining sulfuric acid, the reaction mixture is centrifuged at 13000rmp for 10 minutes, the upper layer solution is filtered, the filtrate is evaporated to dryness under reduced pressure, the crude product is dissolved in chloroform, washed with 10% sodium carbonate solution and saturated brine, and dried over magnesium sulfate , the solvent was distilled off under reduced pressure to obtain 17.31 g of a colorless oily pure product, with a yield of 72.60%. 1 H NMR (400MHz, CDCl 3 ,δ,ppm):0.82–0.86(m,3H...
Embodiment 2
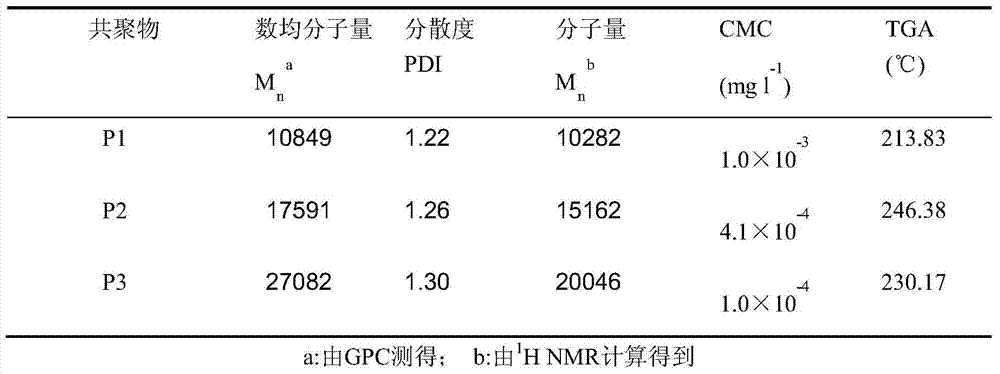
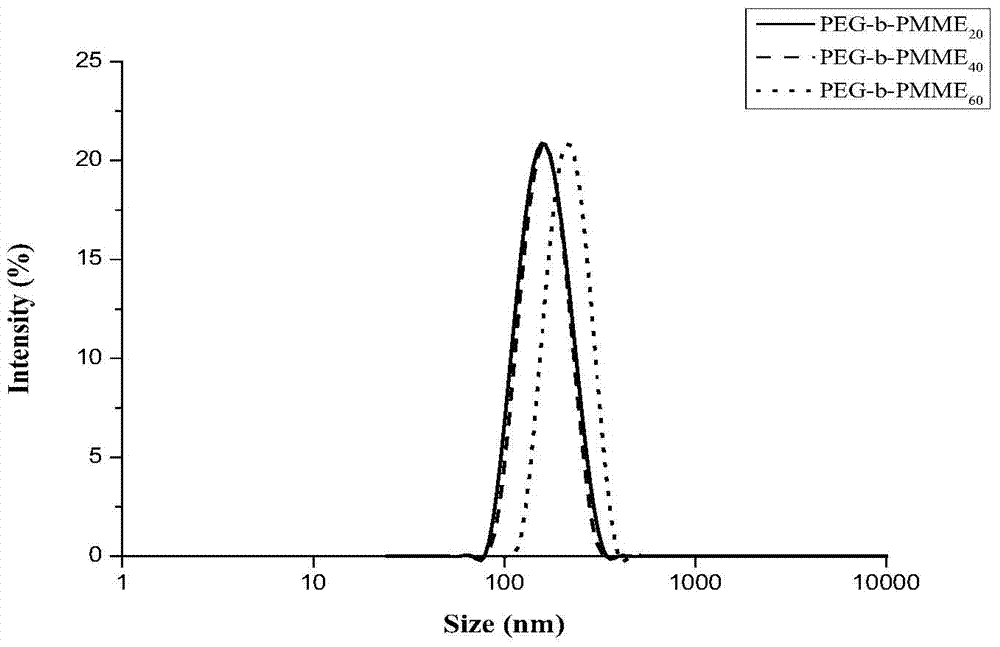
[0045] 1.00g (4.10mmoL) of 2-methoxy-5-ethyl-[1,3]-dioxane-5-acetoacetate methyl ester, 0.74g (0.137mmoL) of polyethylene glycol macroinitiator , 1.12mg (0.0274mmoL) of 2,2-azobisisobutylcyanide was accurately weighed and put into a dry and clean glass polymerization tube, then N,N-dimethylformamide (2.0mL) was added, and the polymerization system was ventilated with nitrogen Seal the tube after degassing for 30 minutes, put the polymerization reaction tube into an oil bath at 70°C to heat and polymerize, and after 24 hours of reaction, dissolve the reaction mixture in N,N-dimethylformamide, then add it dropwise into glacial ether, and collect Precipitate, then rinse with a small amount of glacial ether, the product is vacuum-dried to constant weight to obtain the target copolymer P1, the polymer properties see figure 2 .
Embodiment 3
[0047] Add 0.50g (2.05mmoL) of 2-methoxy-5-ethyl-[1,3]-dioxane-5-acetoacetate methyl ester, 0.18g (0.0341mmoL) of polyethylene glycol macroinitiator , 1.12mg of 2,2-azobisisobutylcyanide (6.80×10-4mmoL) was accurately weighed and put into a dry and clean glass polymerization tube, then N,N-dimethylformamide (1.5mL) was added to polymerize After degassing the system with nitrogen for 30 minutes, seal the tube, put the polymerization reaction tube into an oil bath at 70°C to heat and polymerize, and after 24 hours of reaction, dissolve the reaction mixture in N,N-dimethylformamide, and then add glacial ether dropwise In the process, the sediment was collected, rinsed with a small amount of glacial ether, and the product was vacuum-dried to constant weight to obtain the target copolymer P2. The properties of the polymer are shown in figure 2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com