Synthesis method and application of a kind of methacrylamide orthoester monomer and acid-sensitive amphiphilic block copolymer thereof
A technology of methacrylamide ortho acid and amphiphilic block, which is applied in the directions of organic chemistry, emulsion delivery, etc., can solve the problems of reducing human immunity, delayed treatment, slow release, etc., and achieves good application prospects and good acidity. effect of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
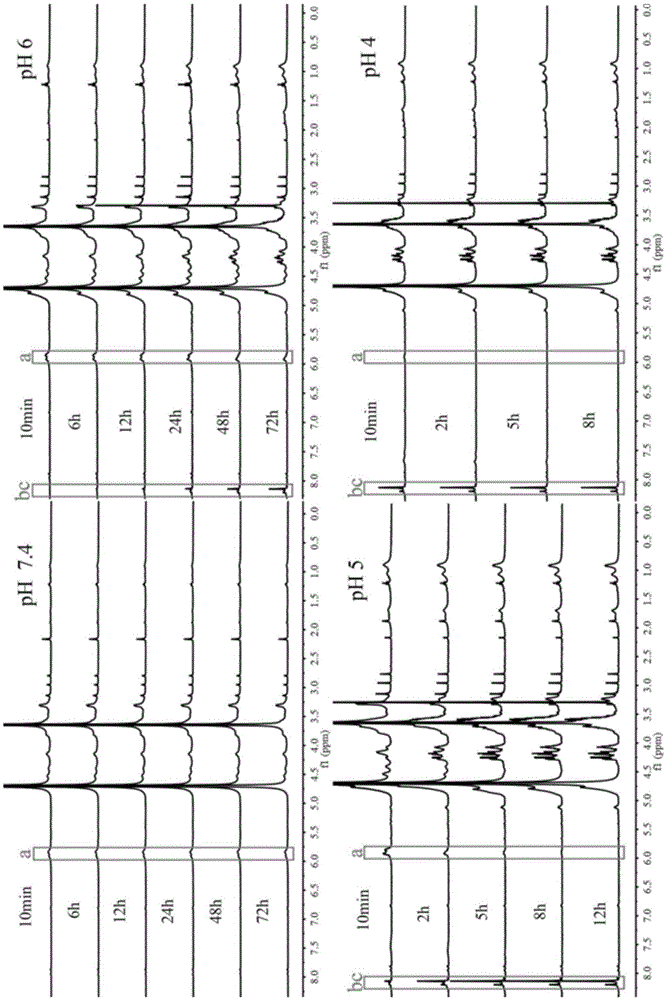
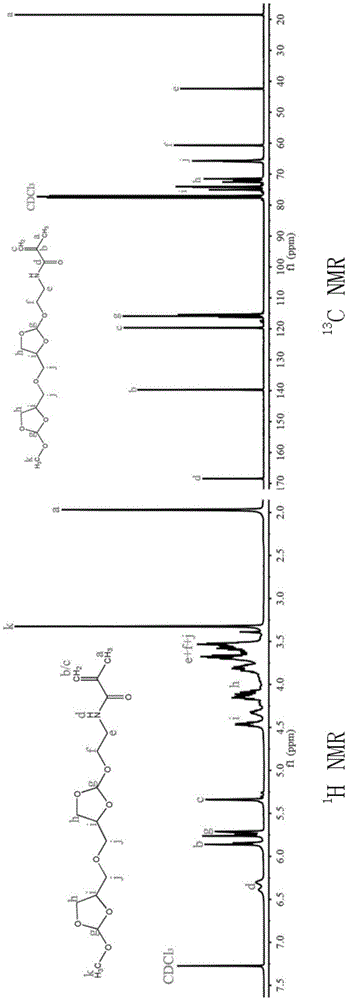
[0039] N-{2-[2-Methoxy-[1,3]dioxolane-4-(2-methoxy-[1,3]dioxolane-4-methyleneoxy)]- The synthetic method of ethyl}-2-methacrylamide monomer is realized through the following steps:
[0040] (1) 2,2,2-Trifluoro-N-{2-methoxy-[1,3]dioxolane-2-[4-(2-methoxy-[1,3]dioxo Preparation of pentane-4-methyleneoxy)]-ethyl}-acetamide:
[0041] Under the action of pyridine-p-toluenesulfonate, 4,4'-dimethyloxy-bis-(2-methoxy-1,3-dioxolane) and N-(2-hydroxyethyl ) trifluoroacetamide reaction, the molar ratio of which is 1:1-1.2, reacted at 130°C for 6h; the reaction system was dissolved in ethyl acetate, washed with 10% sodium carbonate solution, and dried to obtain pure compound 2,2,2- Trifluoro-N-{2-methoxy-[1,3]dioxolane-2-[4-(2-methoxy-[1,3]dioxolane-4-methyleneoxy )]-ethyl}-acetamide, directly used in the next step reaction. 1 HNMR (400MHz, CDCl 3 ,δ,ppm):2.0-2.18(m,NH 2 -CH 2 )3.27-3.28 (m,3H,O-CH 3 ),3.43-3.710(m,6H,CH—
[0042] CH 2 -CH,N-CH 2 -CH 2 ),3.68-4.13(m,4H,O-CH 2...
Embodiment 2
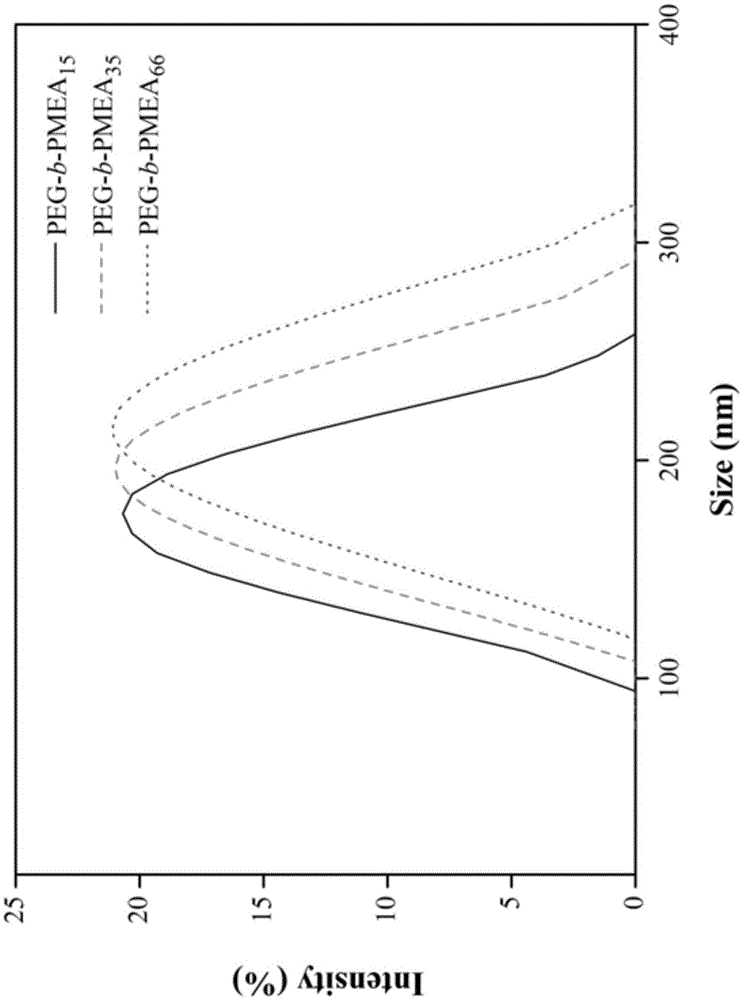
[0048] 1.00g (2.88mmoL) N-{2-[2-methoxy-[1,3]dioxolane-4-(2-methoxy-[1,3]dioxolane-4- Methylene oxide)]-ethyl}-2-methacrylamide, polyethylene glycol macroinitiator 0.52g (0.096mmoL), 2,2-azobisisobutylcyanide 3.15mg (0.0192mmoL) is accurate Weigh it into a dry and clean glass polymerization tube, then add N,N-dimethylformamide (2.5mL), degas the polymerization system with nitrogen for 30 minutes, seal the tube, and put the polymerization reaction tube in an oil bath at 70°C Heating and polymerizing, after 24 hours of reaction, the reaction mixture was dissolved in N,N-dimethylformamide, then added dropwise into glacial ether, the sediment was collected, rinsed with a small amount of glacial ether, and the product was vacuum-dried to constant weight to obtain The target copolymer P1, the properties of the polymer are shown in Table 1.
Embodiment 3
[0050] 1g (2.88mmoL) N-{2-[2-methoxy-[1,3]dioxolane-4-(2-methoxy-[1,3]dioxolane-4- Methyl oxygen)]-ethyl}-2-methacrylamide, polyethylene glycol macroinitiator 0.26g (0.048mmoL), 2,2-azobisisobutyrocyanide 1.58mg (9.6×10 -3 mmoL) was accurately weighed into a dry and clean glass polymerization tube, then N,N-dimethylformamide (2.5mL) was added, the polymerization system was degassed with nitrogen for 30 minutes, and then the tube was sealed, and the polymerization reaction tube was placed at 70°C Heated and polymerized in an oil bath. After 24 hours of reaction, the reaction mixture was dissolved in N,N-dimethylformamide, then added dropwise into glacial ether, the sediment was collected, rinsed with a small amount of glacial ether, and the product was vacuum-dried to The target copolymer P2 was obtained by constant weight, and the properties of the polymer are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com