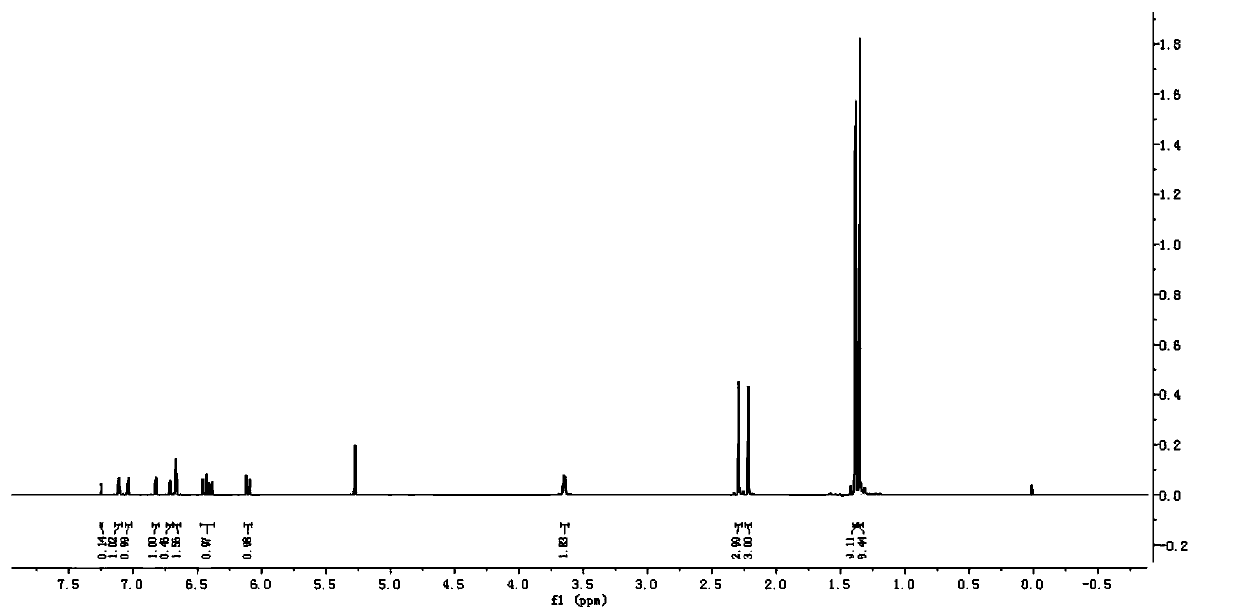
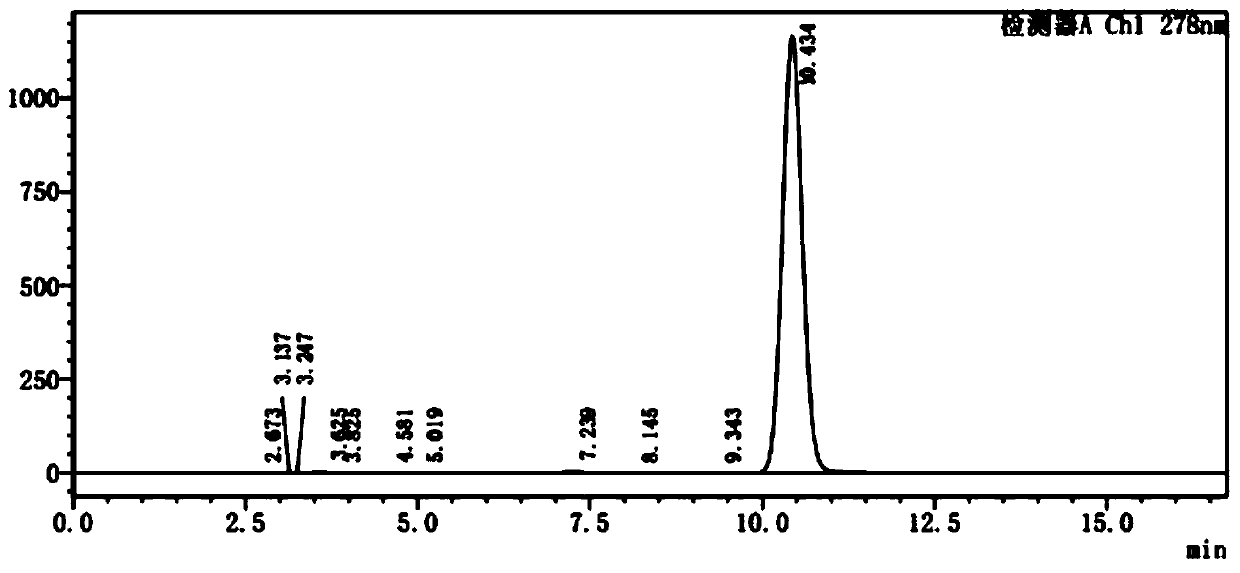
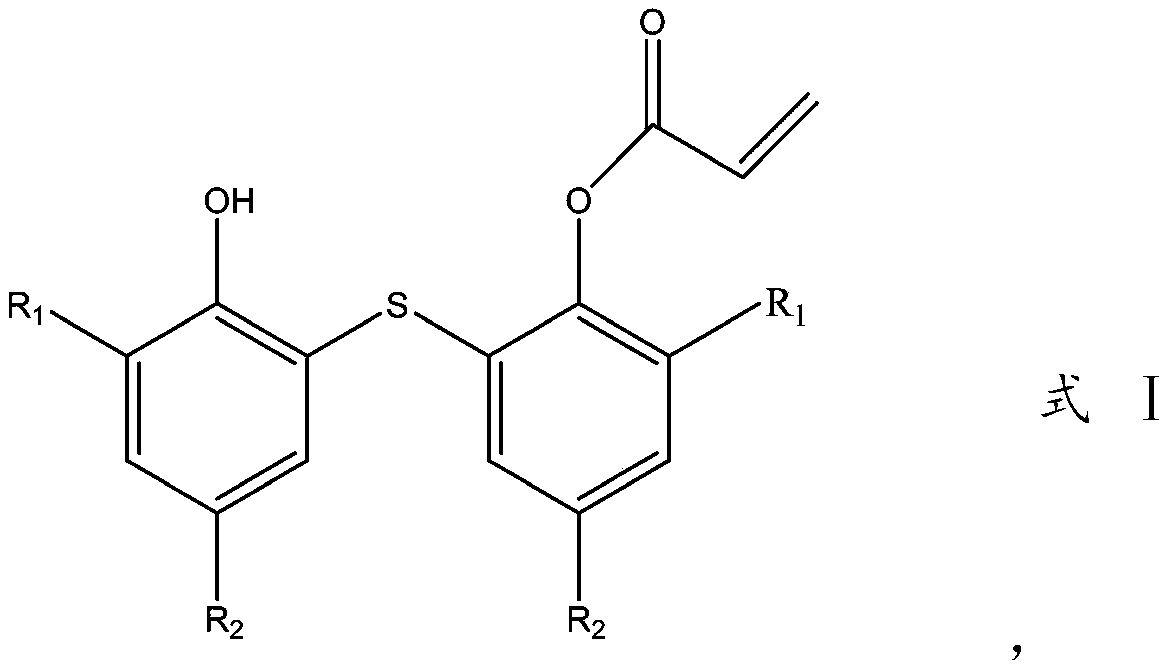
Thioether bisphenol acrylate multi-effect antioxidant and preparation method thereof
A bisphenol acrylate and antioxidant technology, applied in the field of plastic additives, can solve the problem of single anti-aging effect, and achieve the effects of avoiding degradation and aging, low reaction temperature and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The synthesis of embodiment 1 compound a
[0017] Add 358.5g (1mol) of 2,2'-thiobis(4-methyl-6-tert-butylphenol) into a 3L three-necked flask, then add 1000ml of n-heptane, 404g (4mol) of triethylamine, acrylic acid 86.5g (1.2mol), stir and raise the temperature to 60°C, start to add 199g (1.3mol) of phosphorus oxychloride dropwise, control the temperature at 60-80°C during the dropwise addition, add the time for 1h, raise the temperature to 85°C and keep it for 1.5 h. After the reaction, wait for the reaction solution to cool slightly and suction filter, the filter cake is triethylamine salt, rinse with 500ml of n-heptane, combine the organic phases, distill out 1200ml of n-heptane under reduced pressure, and stir the remaining liquid to cool down and crystallize , crystallized at 0 to 5° C., and filtered with suction to obtain 338.3 g of the product with a yield of 82%.
Embodiment 2
[0018] The synthesis of embodiment 2 compound b
[0019] Add 386.5g (1mol) of 2,2'-thiobis(4-ethyl-6-tert-butylphenol) into a 3L three-necked flask, then add 1100ml of n-heptane, 505g (5mol) of triethylamine, acrylic acid 93.7g (1.3mol), stir and heat up to 65°C, start dropwise adding 199g (1.3mol) of phosphorus oxychloride, control the temperature at 65-80°C during the dropwise addition, add dropwise for 1h, raise the temperature to 85°C and keep for 1.5 h. After the reaction is finished, wait for the reaction solution to cool slightly and filter with suction. The filter cake is triethylamine salt, rinse with 500ml of n-heptane, combine the organic phases, distill out 1300ml of n-heptane under reduced pressure, and stir the remaining liquid to cool down and crystallize. , crystallized at 2 to 5° C. to obtain 373 g of the product with a yield of 84.6%.
Embodiment example 3
[0020] The synthesis of embodiment case 3 compound c
[0021] Add 414.5g (1mol) of 2,2'-thiobis(4,6-di-tert-butylphenol) into a 3L three-necked flask, then add 1100ml of toluene, 535g (5.3mol) of triethylamine, and 93.7g of acrylic acid ( 1.3mol), stirred and raised the temperature to 60°C, began to dropwise add 207g (1.35mol) of phosphorus oxychloride, the temperature was controlled at 65-85°C during the dropwise addition, the dropwise time was 1h, and the temperature was raised to 90°C for 1.5h after the dropwise addition. After the reaction, wait for the reaction solution to cool slightly and filter with suction. The filter cake is triethylamine salt, rinse with 600ml of toluene, combine the organic phases, distill 1200ml of toluene under reduced pressure, and stir the remaining residual solution to cool down and crystallize. 5 to 10 Crystallized at ℃ to obtain 414.6 g of the product with a yield of 88.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com