Method for controlling apoptosis and its application in inhibiting tumor growth
A control method and cell generation technology, applied in the fields of biotechnology and medicine, can solve the problems of inability to obtain further application, penetrating cell damage, etc., and achieve the effect of inhibiting growth and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
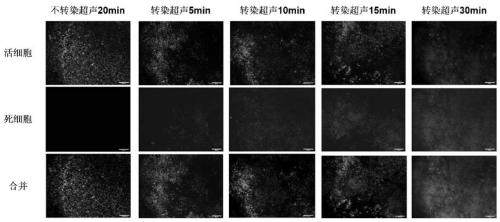
[0035] Hela cells of appropriate density were evenly spread into 12-well plates and cultured for 24 hours, transfected with MscL plasmid, and after 48 hours, the expression of the target protein was verified by a fluorescence microscope, and then fresh culture medium was replaced, and ultrasonic radiation with a power of 15W and a frequency of 6MHz was carried out. The time is 30 minutes. After continuing to culture for 24 hours, the cells were washed with phosphate buffered saline, and then stained with Calcein-AM / PI mixed fluorescent dye for dead and alive cells for 10 minutes, and finally flow cytometry was performed to detect that the apoptosis rate was about 60%.
Embodiment 2
[0037] 4T1 cells with appropriate density were evenly spread into 12-well plates and cultured for 24 hours, transfected with MscL plasmid, and after 48 hours, the expression of the target protein was verified by a fluorescent microscope, and then fresh culture medium was replaced, and ultrasonic radiation with a power of 15W and a frequency of 6MHz was carried out. The time is 60 minutes. After continuing to culture for 24 hours, the cells were washed with phosphate buffered saline, and then stained with Calcein-AM / PI mixed fluorescent dye for dead and alive cells for 10 minutes. Finally, flow cytometry was performed to detect that the apoptosis rate was about 80%.
Embodiment 3
[0039] A subcutaneous model of breast cancer in mice was constructed, and when the tumor diameter reached about 4-6 mm, group treatment began. They were divided into 4 groups with 5 mice in each group. The specific treatment plan is as follows:
[0040] Group 1: Local injection of phosphate buffered saline
[0041] Group 2: local injection of phosphate buffer, and ultrasonic treatment for 7 days.
[0042] Group 3: local injection of in vivo transfection reagent carrying MscL plasmid.
[0043] Group 4: local injection of in vivo transfection reagent carrying MscL plasmid, and ultrasonic treatment for 7 days. figure 1 Cell apoptosis after ultrasonic radiation for different time.
[0044] After the treatment, the body weight and tumor volume of the mice were measured every day. The tumor volume of the mice in the fourth group was significantly smaller than that of the other three groups, which can effectively inhibit the growth of the tumor.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com