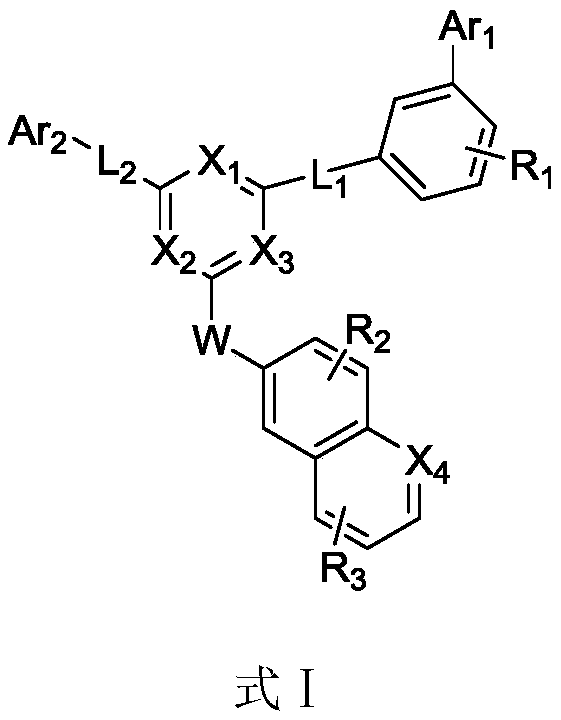
Heterocyclic organic photoelectric material, and preparation method and application thereof
A technology of organic optoelectronic materials and heterocyclic rings, applied in the direction of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of unsatisfactory device driving voltage luminous efficiency, etc., and achieve excellent electron transport characteristics, excellent hole mobility, The effect of high luminous power
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1: Compound 1 and its synthetic method
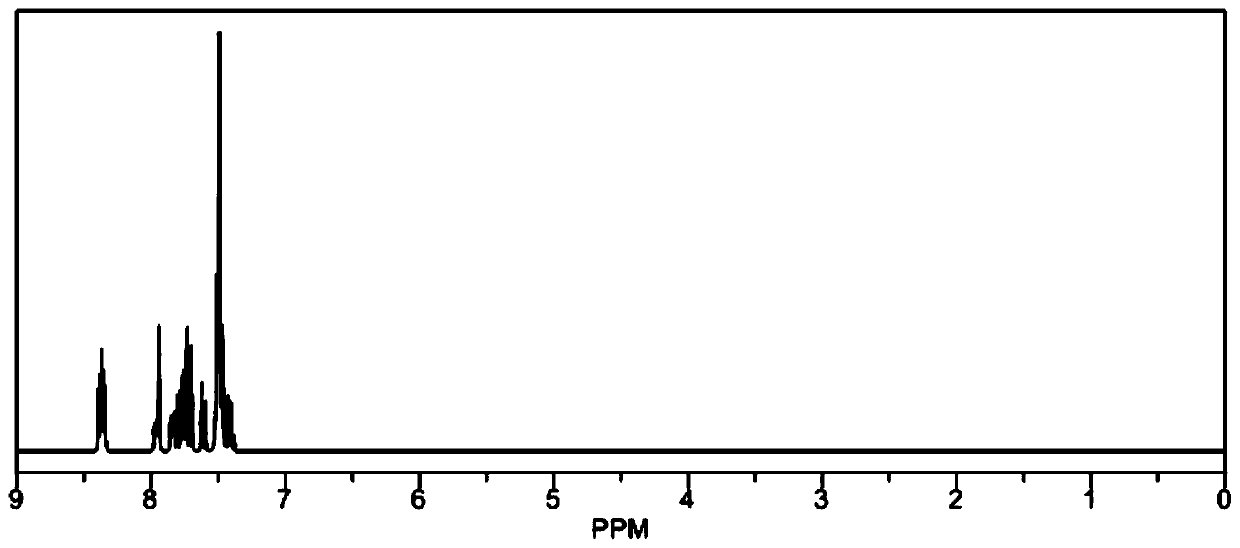
[0069] The structure of compound 1 is as follows:
[0070] The synthetic method of above-mentioned compound 1, according to following synthetic method:
[0071] Add 0.2mol of raw material 1a to a 2L three-necked flask, then measure 400.00ml of DMF, dissolve the raw material under mechanical stirring, slowly add 0.3mol of sodium hydride, and start to drop the DMF solution of raw material 1b (0.2mol +200mlDMF) The reaction temperature was 5°C, the reaction time was 2 hours, the liquid phase followed the reaction of the raw materials, and the reaction was terminated.
[0072] After the reaction is over, dilute the reaction solution with 1.5L of dichloroethane and slowly pour it into 3L of water, let it stand, separate the liquids, extract the water phase with 1L of dichloroethane, combine the organic phases, wash with water until neutral, and use After drying 200g of anhydrous sodium sulfate, distill under reduced pre...
Embodiment 2
[0080] Embodiment 2: Compound 2 and its synthetic method
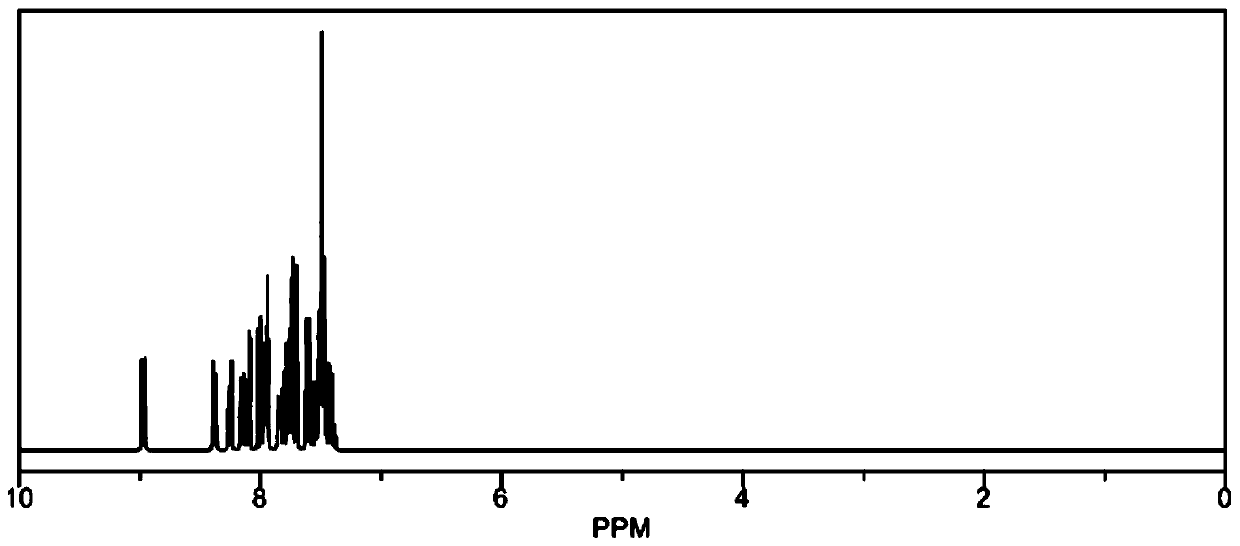
[0081] The structure of compound 2 is as follows:
[0082] The synthetic method of above-mentioned compound 2, according to following synthetic method:
[0083] Add 0.2 mol of raw material 2a to a 2L three-necked flask, then measure 480.00ml of DMF, and dissolve the raw material under mechanical stirring, slowly add 0.4 mol of sodium hydride, and dropwise add the DMF solution of raw material 2b (0.22mol+135mlDMF). The reaction temperature is 15 °C, the reaction time was 5 hours, the liquid phase followed the reaction of the raw materials, and the reaction was terminated.
[0084] After the reaction is over, dilute the reaction solution with 1.5L of dichloroethane and slowly pour it into 3L of water, let it stand, separate the liquids, extract the water phase with 1L of dichloroethane, combine the organic phases, wash with water until neutral, and use After drying 200g of anhydrous sodium sulfate, distill under redu...
Embodiment 3
[0090] Embodiment 3: Compound 3 and its synthetic method
[0091] The structure of compound 3 is as follows:
[0092] The synthetic method of above-mentioned compound 3, according to following synthetic method:
[0093]Add 0.2 mol of raw material 3a to a 2L three-necked flask, then measure 500.00ml of DMF, and dissolve the raw material under mechanical stirring, slowly add 0.5 mol of sodium hydride, and dropwise add the DMF solution of raw material 2b (0.24mol+200mlDMF). The reaction temperature is 25 °C, the reaction time is 10 hours, the liquid phase follows the reaction of the raw materials, and the reaction is terminated.
[0094] After the reaction is over, dilute the reaction solution with 1.5L of dichloroethane and slowly pour it into 3L of water, let it stand, separate the liquids, extract the water phase with 1L of dichloroethane, combine the organic phases, wash with water until neutral, and use After drying 200g of anhydrous sodium sulfate, distill under reduced...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com