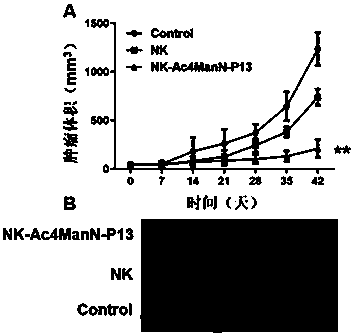
Preparation method for membrane surface engineered NK cell, pharmaceutical composition and application
A NK cell and engineering technology, applied in drug combinations, animal cells, vertebrate cells, etc., can solve the problems of non-response to treatment, low transfection efficiency of NK cell viral vector, etc., to enhance the therapeutic effect and enhance tumor infiltration ability. , the effect of eliminating potentially dangerous
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of NK-Ac4ManN-P13 cells includes the following steps
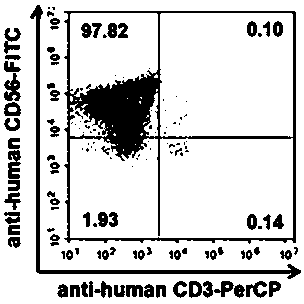
[0025] 1.1 NK cells are obtained by in vitro expansion and culture of peripheral blood mononuclear cells, such as figure 1 As shown in, the NK cells (CD3 - CD56 + ) purity up to 95%;
[0026] 1.2 Use L500 medium to adjust the concentration of NK cells to 1×10 6 -3×10 6 a / mL;
[0027] 1.3 Add the targeting carrier Ac4ManNAz or Ac4ManN-DBCO at a final concentration of 26-60 μM, and set the temperature at 37°C with 5% CO 2 Incubate in an incubator for 12-48 hours;
[0028] 1.4 After the incubation, centrifuge at room temperature for 5 minutes at a centrifugal force of 400×g, discard the supernatant, resuspend the cells in L500 medium, and add PD-L1-targeting polypeptide N3-P13 or DBCO-P13 at a final concentration of 10-40 μM Peptides, under the set conditions at a temperature of 37°C containing 5% CO 2 Secondary incubation in the incubator for 0.5-2 hours;
[0029] 1.5 After the second in...
Embodiment 2
[0030] Example 2 Positive detection of NK-Ac4ManN-P13 cells
[0031] 2.1 Prepare NK-Ac4ManN-P13 cells according to the preparation method in Example 1, replace the N3-P13 or DBCO-P13 polypeptide targeting PD-L1 in step 1.4 with N3-P13-FITC or DBCO-P13-FITC polypeptide, and prepare NK-Ac4ManN-P13-FITC cells;
[0032] 2.2 The results of detecting the proportion of NK-Ac4ManN-P13-FITC cells by confocal fluorescence microscopy are as follows: figure 2 As shown in -A, the results of detecting the ratio of NK-Ac4ManN-P13-FITC cells by flow cytometry are as follows figure 2 As shown in -B, the proportion of NK-Ac4ManN-P13-FITC cells reached 100%.
Embodiment 3
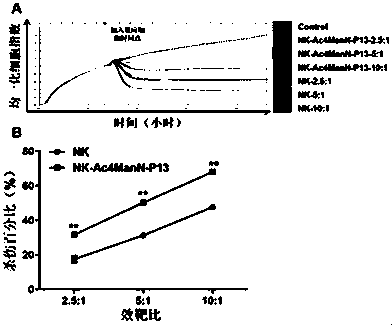
[0033] Example 3 In vitro killing activity detection of NK-Ac4ManN-P13 cells
[0034] Detect the killing activity of NK-Ac4ManN-P13 cells prepared in Example 1
[0035]The killing activity of NK-Ac4ManN-P13 cells and control cells with different effect-to-target ratios on FaDu cells of head and neck squamous cell carcinoma was detected by using the real-time label-free cell function analyzer of Essen Bio.
[0036] The specific operation steps are as follows:
[0037] 3.1 Take the FaDu cells in the logarithmic growth phase, and routinely digest them to a concentration of 2.67×10 5 cell suspension per mL;
[0038] 3.2 Add the cell suspension obtained in step 3.1 to the real-time label-free cell function analyzer E-Plate 8 detection plate, add 300 μL to each well, and place in 37°C containing 5% CO 2 Incubate in the incubator for 18-24 hours;
[0039] 3.3 Add 100 μL of NK-Ac4ManN-P13 cell suspension and control group NK cell suspension to E-Plate 8 with different effect-to-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com