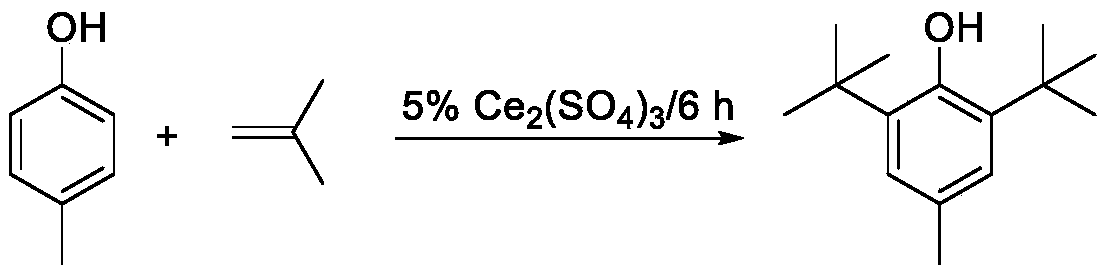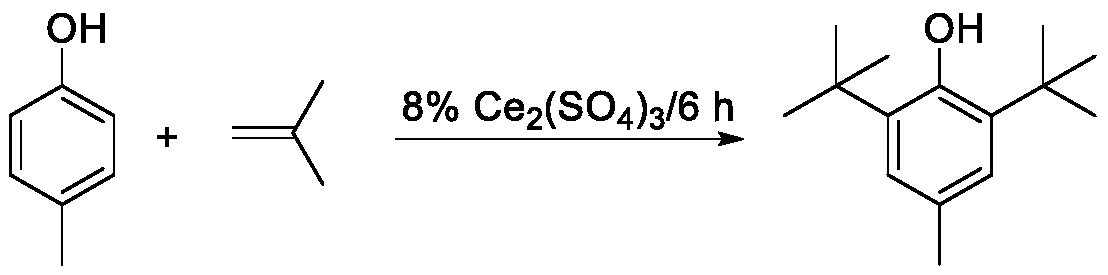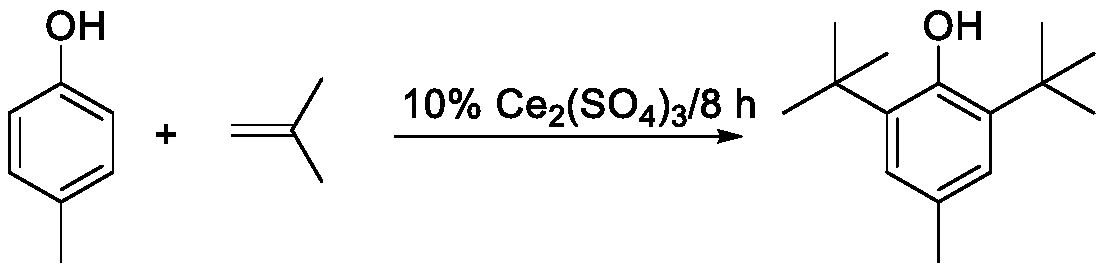New process for preparing food-grade 2,6-di-tert-butyl-p-methylphenol
A technology of p-cresol and di-tert-butyl, which is applied in the new process field of preparing food-grade 2,6-di-tert-butyl-p-cresol, which can solve the problems of long reaction time, low conversion efficiency and large amount of waste water , to achieve the effect of convenient purification and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Use 10g of solid acid catalyst cerium sulfate, 200g of p-cresol, the mass ratio of catalyst to p-cresol is 5%, the reaction temperature is 90-110°C, after the temperature in the flask reaches the set value, start to pass isobutylene, the reaction time is 6h, and the catalyst is separated and removed According to gas chromatography analysis, the content of 2,6-di-tert-butyl-p-cresol in the alkylation liquid is 87.21%, and after recrystallization and drying, the purity reaches 99.22%.
[0017] Described alkylation reaction equation is:
[0018]
Embodiment 2
[0020] 16g of cerium sulfate as a solid acid catalyst, 200g of p-cresol, 8% mass ratio of catalyst to p-cresol, and a reaction temperature of 90-110°C were used. After the temperature in the flask reached the set value, isobutylene was started to flow for a reaction time of 6h. The catalyst was separated and removed, and analyzed by gas chromatography, the content of 2,6-di-tert-butyl-p-methylphenol in the alkylation liquid was 85.8%, and after recrystallization and drying, the purity reached 99.3%.
[0021] Described alkylation reaction equation is:
[0022]
Embodiment 3
[0024] Use 20g of cerium sulfate as a solid acid catalyst, 200g of p-cresol, 10% mass ratio of catalyst to p-cresol, and a reaction temperature of 90-110°C. After the temperature in the flask reaches the set value, start to pass isobutylene for a reaction time of 8 hours. The catalyst was separated and removed, and analyzed by gas chromatography, the content of 2,6-di-tert-butyl-p-methylphenol in the alkylation liquid was 86.6%, and after recrystallization and drying, the purity reached 99.7%.
[0025] Described alkylation reaction equation is:
[0026]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com