CAR-T in treatment of hepatocellular carcinama by targeting HBV and application thereof
A targeting and chimeric antigen receptor technology, applied in the field of immunotherapy, can solve the problem of unavoidable off-target effects in multiple organs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Construction of vector expressing CAR lentivirus and preparation of CAR-T cells
[0039] 1. Experimental method
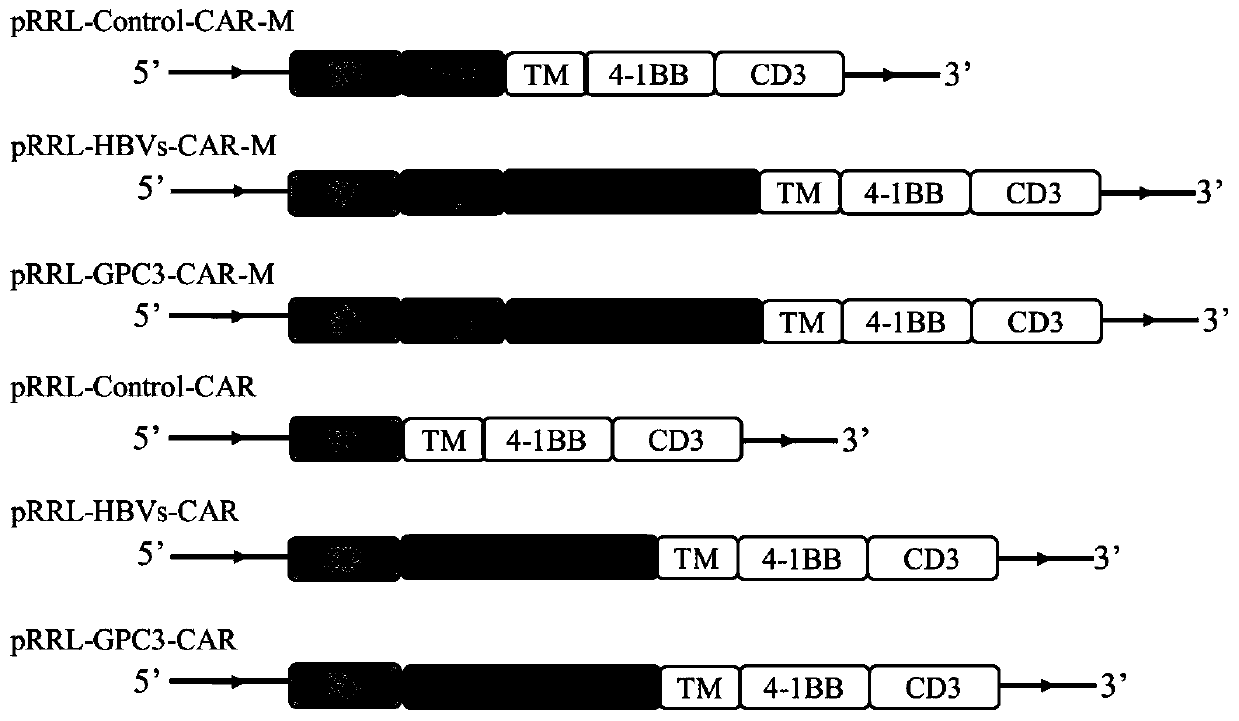
[0040] (1) Gene synthesis is a DNA fragment composed of encoding CD8α signal peptide (SP), myc tag, CD8 hinge and CD8 transmembrane region (TM), 4-1BB and CD3ζ intracellular signal domain in series, and the upstream and downstream Add BamHI and SalI restriction sites, respectively, and insert the synthetic gene into the pRRL vector after double digestion to construct the plasmid pRRL-Control-CAR-M.
[0041] (2) Gene synthesis is composed of DNA fragments in series encoding CD8α signal peptide, myc tag, anti-HBVs scFv, CD8 hinge and CD8 transmembrane region, 4-1BB and CD3ζ intracellular signal domains, and the upstream and downstream are respectively added BamHI and SalI restriction sites, the synthetic gene was inserted into the pRRL vector after double digestion to construct the plasmid pRRL-HBVs-CAR-M.
Embodiment 2
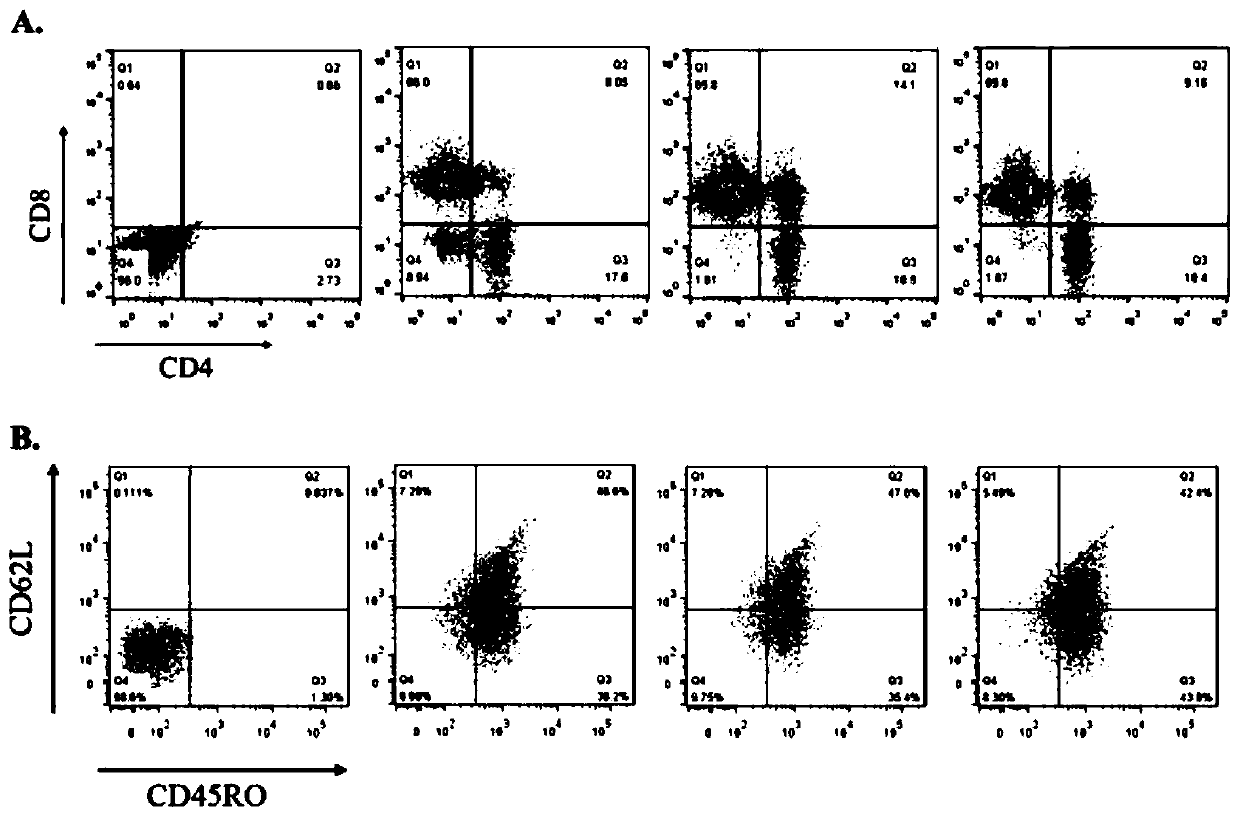
[0051] Example 2 Phenotype detection of CAR-T cells
[0052] 1. Experimental method
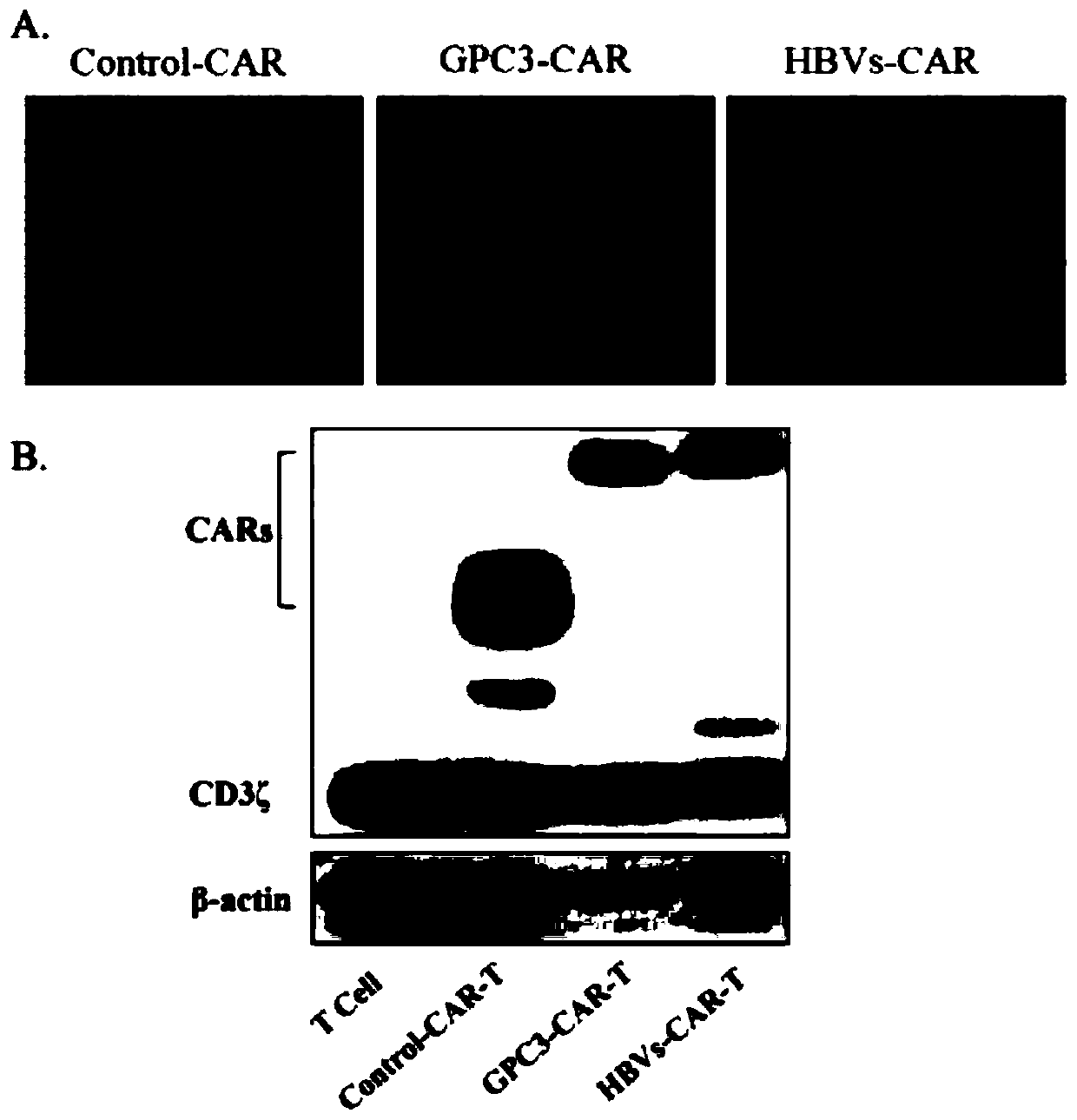
[0053] (1) Each CAR-T cell after lentivirus infection was cultured for 10 days, and 1×10 6 Total cell protein was extracted from each cell. After the protein was separated by SDS-PAGE gel, it was transferred to the membrane, and the expression of each CAR was detected by CD3ζ chain antibody and tag Flag or myc antibody.
[0054] (2) After the CAR-T cells infected with lentivirus continued to be cultured for 10 days, 1×10 5 After being fixed with 4% paraformaldehyde, the cells were incubated with myc antibody and labeled with FITC-labeled secondary antibody. After centrifugation, the cells were transferred to glass slides, and then the cell localization of each CAR was observed by confocal laser microscopy.
[0055] (3) After each CAR-T cell was cultured for 10 days after lentivirus infection, 1×10 5 Cells were incubated with Flag or myc antibody and labeled with secondary antibody to dete...
Embodiment 3
[0058] Example 3 Detection of the in vitro killing ability of each CAR-T cell
[0059] 1. Experimental method
[0060] Subsequent experiments (including in vivo and in vitro) were carried out using the HBVs+GPC3+ cell line HepG2.2.15, the HBVs-GPC3+ cell line HepG2 and the HBVs-GPC3- cell line SK-HEP-1 to comprehensively evaluate each CAR-T cell for its target cell Assess the off-target effects on non-target cells, as follows:
[0061] (1) The proliferation activity of various CAR-T cells was detected by flow cytometry.
[0062] For each CAR-T cell labeled with CFSE, take 1×10 5 labeled cells with 1×10 5 The above three liver cancer cells were co-cultured, and the cells were collected on the 2nd, 4th, 6th, 8th, and 10th day, and the proliferation of each CAR-T cell was analyzed by flow cytometry.
[0063] (2) ELISA detection of cytokine expression of CAR-T cells
[0064] Take 1×10 4 CAR-T cells were mixed with 1×10 4 The above three liver cancer cells were co-cultured, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com