Electrochemical anode preparation method and electrochemical anode
An electrochemical and anode technology, applied in the field of electrolysis of electrolytic cells, can solve the problems of active coating shedding, electrochemical corrosion of titanium substrates, and impact on the service life of titanium anodes, and achieve the effect of avoiding shedding.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] This embodiment provides a method for preparing an electrochemical anode, which includes using a porous metal material as a substrate, preparing an intermediate layer containing metal oxide on the surface of the substrate, and preparing a non-metallic conductive electrode on the surface of the intermediate layer containing metal oxide. The surface layer forms the finished electrochemical anode.
[0019] In the electrochemical anode of this embodiment, the non-metal conductive surface layer is located on the surface layer of the electrochemical anode. Since the chemical dissolution rate of the material mainly depends on the metallicity of the material, for metals, metal oxides and non-metallic materials, the metallicity decreases in turn, and the lower the metallicity, the slower the chemical dissolution rate. Therefore, in order to avoid hydrochloric acid or Sulfuric acid and other acid-containing environments are chemically dissolved. In this embodiment, non-metallic m...
experiment example 1
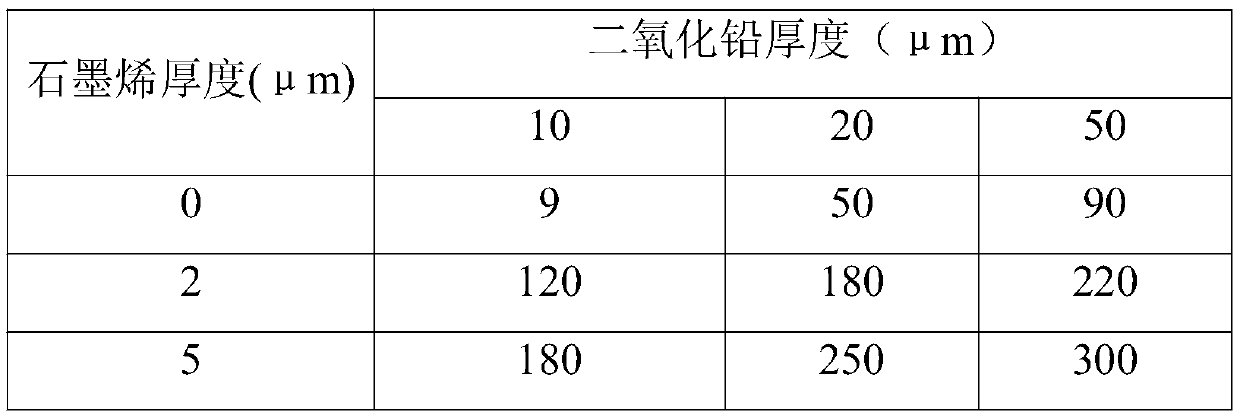
[0025] In this experimental example, a sandblasted titanium plate is used as the substrate, lead dioxide with different thicknesses is used as the middle layer containing metal oxides, and graphene layers with different thicknesses are used as the non-metallic conductive surface layer. 2 The life situation in the accelerated environment, analysis results. details as follows:
[0026] A sandblasting titanium plate with a thickness of 2 mm is selected, that is, the titanium plate is subjected to sandblasting treatment, and the surface of the titanium plate is cleaned after the sandblasting treatment as a base material. Combine lead dioxide and an appropriate amount of organic particles with the substrate through composite electroplating or composite coating or electroplating and coating. The middle layer of the structure to increase the specific surface area and reduce the current density to reduce energy consumption. Finally, 0 μm, 2 μm, and 5 μm graphene were sprayed on the ...
experiment example 2
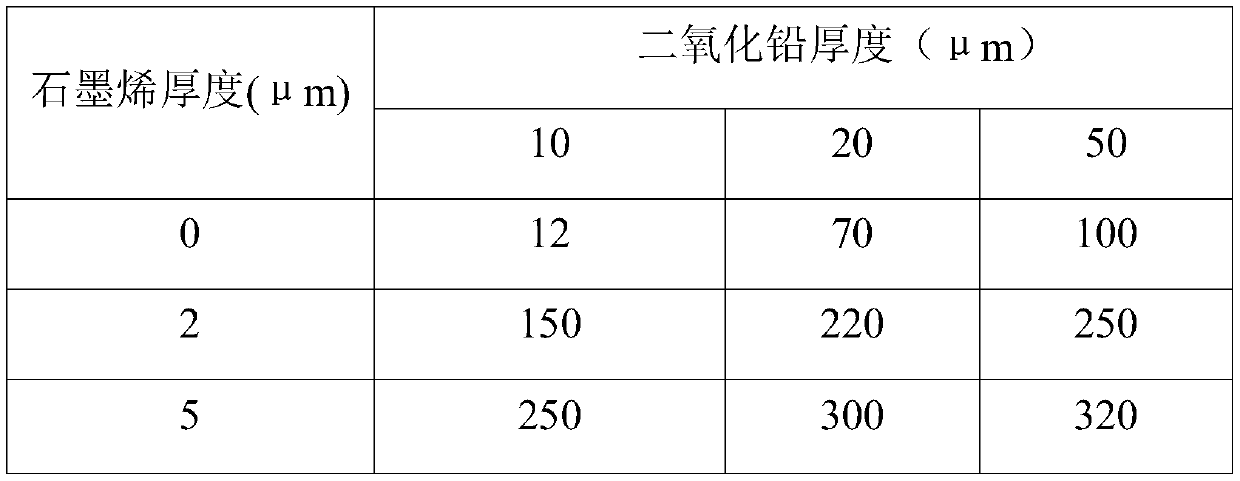
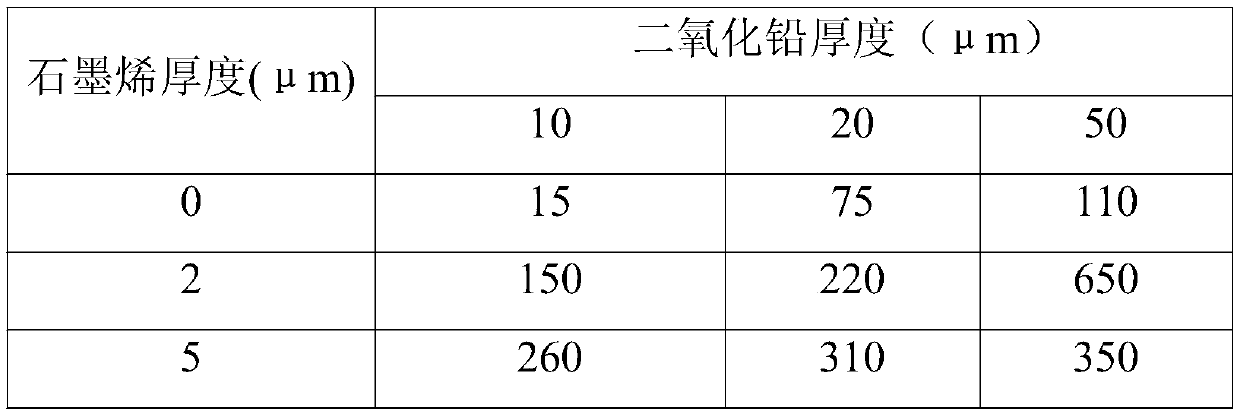
[0031] In this experimental example, the porous titanium plate is used as the base material, the lead dioxide of different thickness is used as the intermediate layer containing metal oxide, and the graphene layer of different thickness is used as the non-metallic conductive surface layer. 2 The life situation in the accelerated environment, analysis results. details as follows:
[0032] A porous titanium plate with a thickness of 2 mm was selected as the substrate. Combine lead dioxide and an appropriate amount of organic particles with the substrate through composite electroplating or composite coating or electroplating and coating. The middle layer of the structure to increase the specific surface area and reduce the current density to reduce energy consumption. Finally, 0 μm, 2 μm, and 5 μm graphene were sprayed on the middle layer of lead dioxide and heated and cured to form a non-metallic conductive surface layer. In 1m sulfuric acid, 2A / cm 2 Test in an accelerated e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com