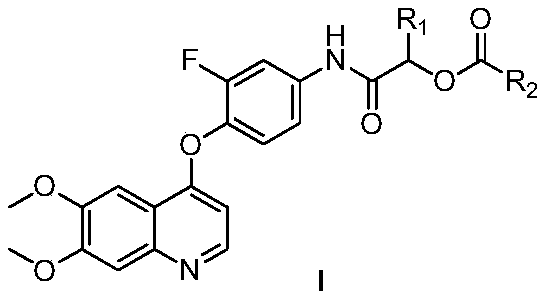
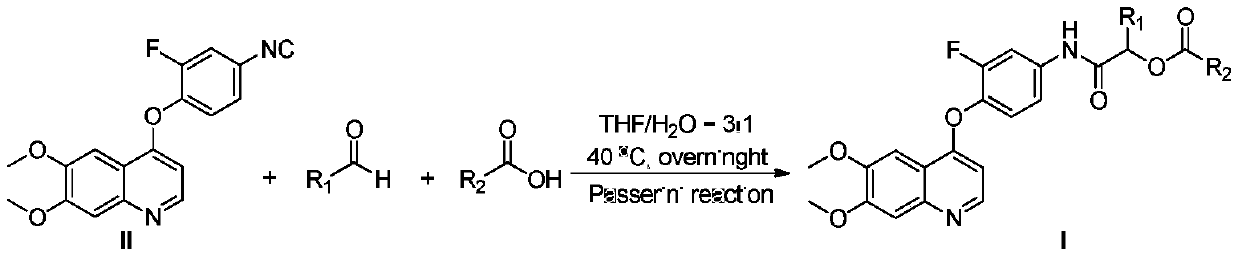
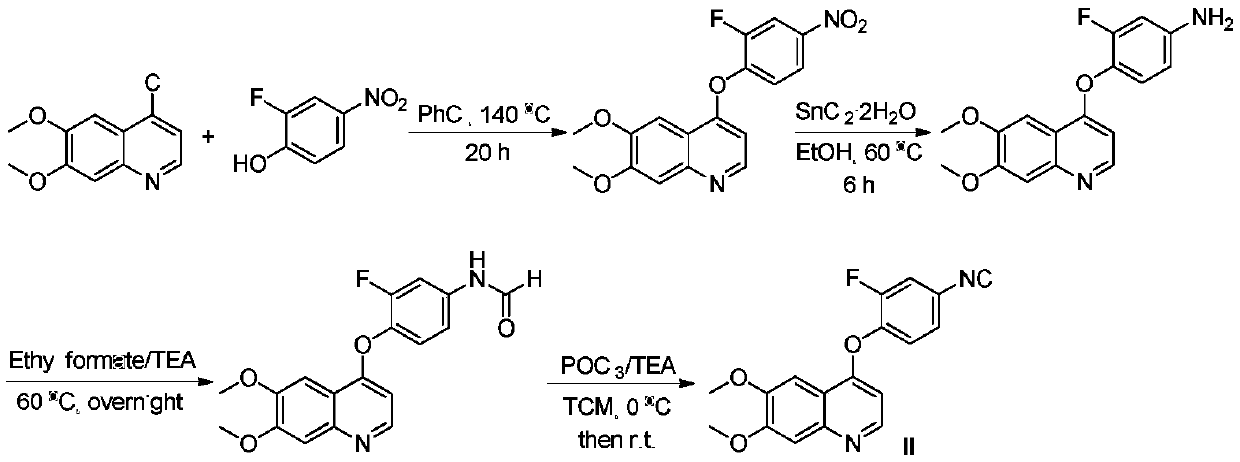
4-phenoxyquinoline and alpha-acyloxyamide compound and preparation method and application thereof
A technology of acyloxyamide and phenoxyquinoline, which is applied in the field of cancer related to c-Met, can solve the problems that have not been reported, and achieve the effect of high purity, strong inhibitory activity and novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the synthesis of target compound Ia
[0035]
[0036] Step 1. The synthesis of 4-(2-fluoro-4-nitrophenyloxy)-6,7-dimethoxyquinoline, the reaction formula is as follows:
[0037]
[0038] Take 4-chloro-6,7-dimethoxyquinoline (6.71g, 30.0mmol) and 2-fluoro4-nitrophenol (5.65g, 36.0mmol) in 60mL of chlorobenzene, and slowly heat to 140°C , Continue to react at this temperature for 20h. Heating was then stopped, cooled to room temperature, and the solvent was evaporated under reduced pressure. The residue was dissolved with dichloromethane, washed with saturated potassium carbonate solution and water successively, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and purified by silica gel column chromatography (PE / EA=3:1), 6.30 g of a light yellow solid was obtained, and the yield was 61%. Mp: 161–163°C.1 H NMR (400MHz, DMSO-d 6 )δ8.56(d, J=5.2Hz, 1H), 8.43(dd, J=2.4, 10.4Hz, 1H), 8.18(d, J=8.8Hz, 1H), 7.60(t, J=8.4Hz, 1H), ...
Embodiment 2
[0051] Embodiment 2: the synthesis of target compound Ib
[0052]
[0053] The experimental procedure was the same as in Example Ia, only benzoic acid was used instead of thiophene-2-carboxylic acid. White solid, yield: 72.4%. Mp:91-93℃. 1 H NMR (400MHz, DMSO-d 6 )δ10.70(s,1H),8.46(d,J=5.6Hz,1H),8.02(d,J=7.2Hz,2H),7.87(dd,J=2.0,12.8Hz,1H),7.68( t,J=7.2Hz,1H),7.56(d,J=7.6Hz,2H),7.53(s,1H),7.50(dd,J=1.6,9.2Hz,1H),7.44(d,J=8.8 Hz, 1H), 7.40(s, 1H), 6.46(d, J=4.8Hz, 1H), 5.31(q, J=6.8Hz, 1H), 3.94(s, 6H), 1.61(d, J=6.8 Hz,3H). 13 C NMR (100MHz, DMSO-d 6 )δ169.2, 165.3, 159.5, 153.5 (d, J = 244.2Hz), 152.8, 149.6, 148.6, 146.1, 137.7 (d, J = 9.9Hz), 135.8 (d, J = 12.3Hz), 133.6, 129.4 (2C ), 129.3, 128.8(2C), 124.2, 116.2(d, J=2.5Hz), 114.5, 108.2(d, J=23.0Hz), 107.6, 102.1, 99.0, 70.8, 55.8(2C), 17.4.ESI- MS: m / z 491.1[M+H] + .
Embodiment 3
[0054] Embodiment 3: the synthesis of target compound Ic
[0055]
[0056] The experimental procedure is the same as in Example Ia, except that n-butyraldehyde is used instead of acetaldehyde, and benzoic acid is used instead of thiophene-2-carboxylic acid. White solid, yield: 68.3%. Mp:99-101℃. 1 H NMR (400MHz, DMSO-d 6)δ10.62(s,1H),8.45(d,J=4.8Hz,1H),8.03(d,J=7.6Hz,2H),7.86(d,J=13.2Hz,1H),7.69(t, J=7.6Hz, 1H), 7.58-7.54(m, 2H), 7.52(s, 1H), 7.48-7.41(m, 2H), 7.39(s, 1H), 6.45(d, J=5.2Hz, 1H ),5.19(t,J=7.2Hz,1H),3.93(s,6H),1.98-1.91(m,2H),1.56-1.49(m,2H),0.97(t,J=7.2Hz,3H) . 13 CNMR (100MHz, DMSO-d 6 )δ168.7, 165.5, 159.4, 153.5 (d, J = 244.3Hz), 152.7, 149.5, 148.9, 146.4, 137.6 (d, J = 9.7Hz), 135.8 (d, J = 12.3Hz), 133.8, 129.5 (2C ),129.2,128.9(2C),124.3,116.2(d,J=1.5Hz),114.6,108.2(d,J=22.6Hz),107.9,102.1,99.0,74.2,55.8(2C),33.4,18.3, 13.7. ESI-MS: m / z 519.2 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com