Positron imaging agent <18>F-FPGalNAc as well as preparation method and application thereof
A positron imaging agent, 18f-fpgalnac technology, which is applied in the field of positron imaging agent 18F-FPGalNAc and its preparation, can solve the problems of developing false negatives in cancer, and achieves strong specificity, improved yield and imaging effect Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
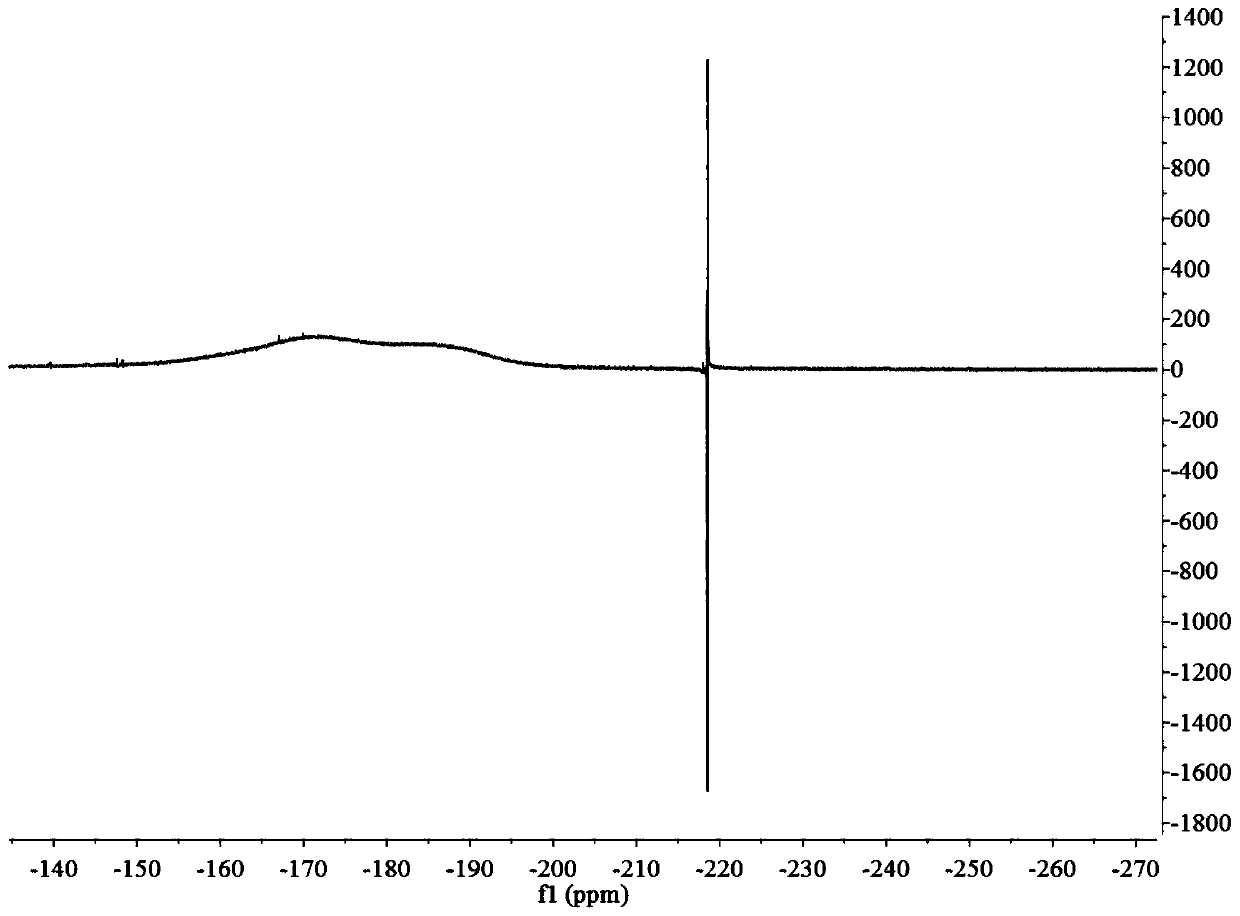
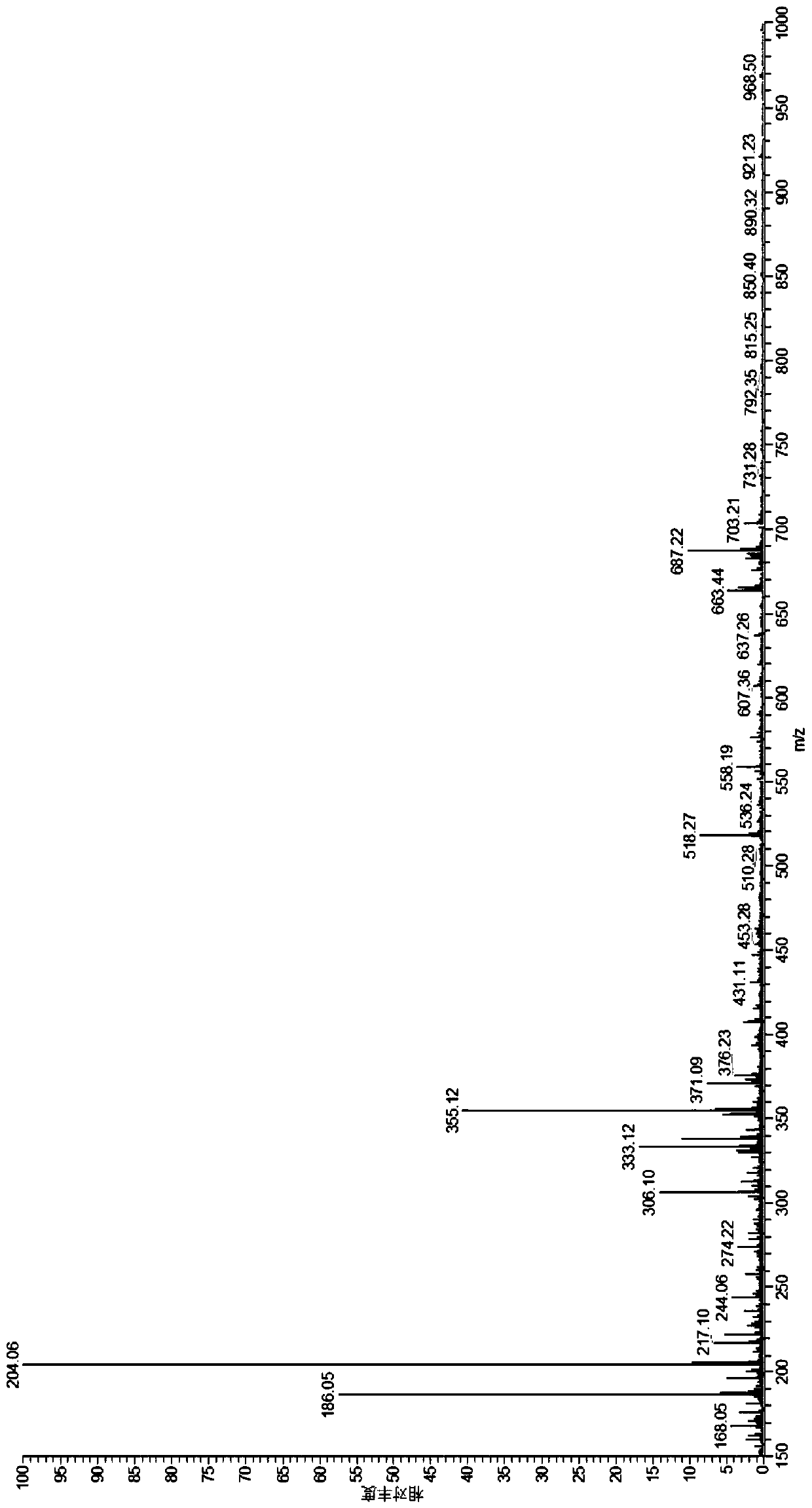
[0055] A positron imaging agent 18 The preparation method of F-FPGalNAc comprises the following steps:
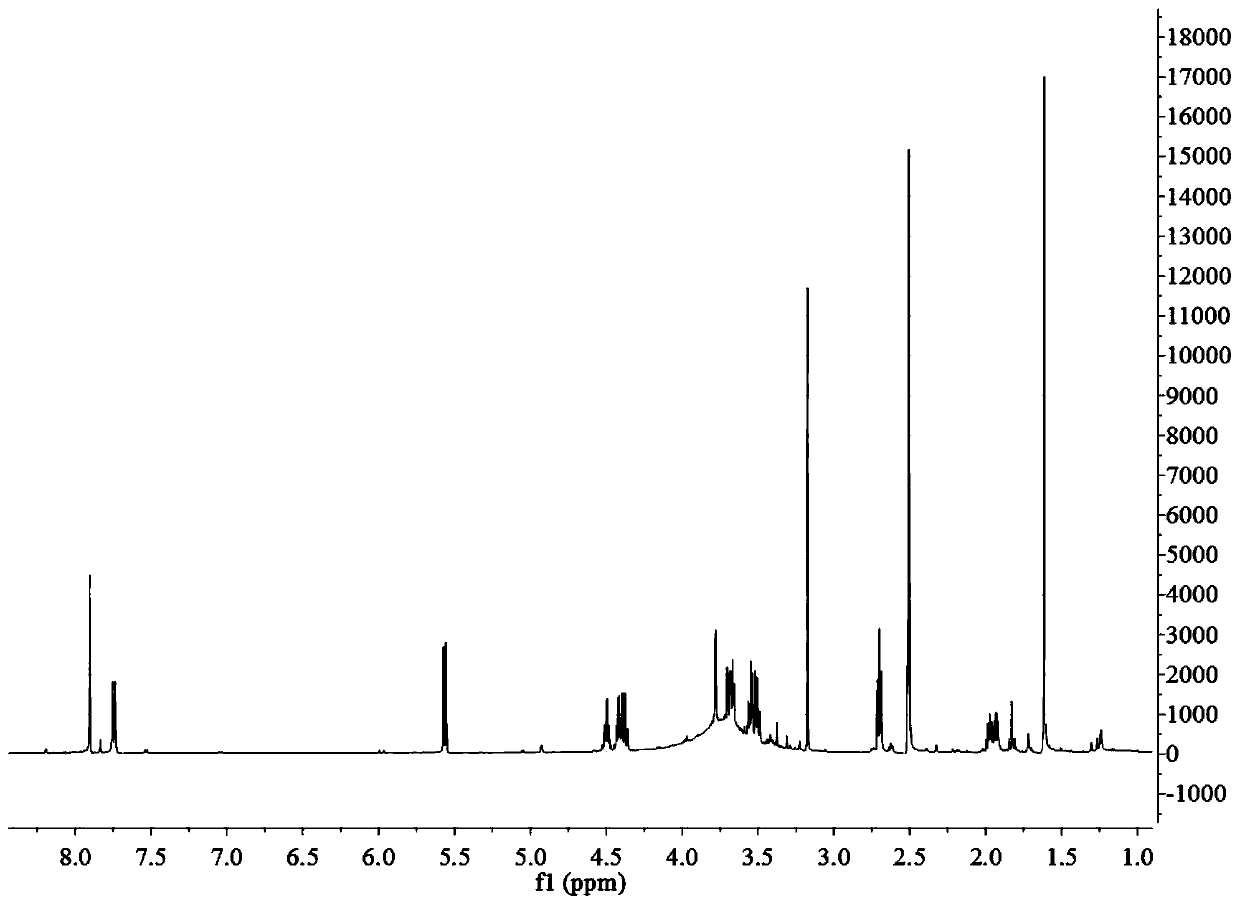
[0056] 1) Disperse 4-pentyn-1-ol (2.52g, 30mmol) in 80mL of dichloromethane, then add triethylamine (10.12g, 100mmol), 4-dimethylaminopyridine (0.16g, 1.32mmol) and p-toluenesulfonyl chloride (6.86g, 36mmol), reacted at room temperature for 14h, added ammonium chloride (5.35g, 0.1mol) to neutralize triethylamine, then extracted with ethyl acetate, and then subjected the extract to anhydrous sodium sulfate Remove water, vacuum distillation and column chromatography to obtain light yellow liquid Yield 83%; 1 H NMR (600MHz, CDCl 3 )δ7.80(d, J=8.3Hz, 2H), 7.35(d, J=8.1Hz, 2H), 4.15(t, J=6.1Hz, 2H), 2.44(d, J=13.4Hz, 3H) ,2.26(dt,J=6.9,2.6Hz,2H),1.86(m,2H),1.23(m,1H);
[0057] 2) Will (35.7mg, 0.15mmol) was dispersed in 1mL acetonitrile, then added 18 F ions (100mCi), reacted at 85°C for 10min to obtain Yield 40%;
[0058] 3) Will (1g, 4.6mmol) was dispersed in 40m...
Embodiment 2
[0071] A positron imaging agent 18 The preparation method of F-FPGalNAc comprises the following steps:
[0072] 1) Disperse 4-pentyn-1-ol (2.52g, 30mmol) in 100mL of dichloromethane, then add triethylamine (10.12g, 100mmol), 4-dimethylaminopyridine (0.16g, 1.32mmol) and p-toluenesulfonyl chloride (6.86g, 36mmol), react at room temperature for 10h, add ammonium chloride (5.35g, 0.1mol) to neutralize triethylamine, extract with ethyl acetate, and then remove the extract with anhydrous sodium sulfate Separated by water, vacuum distillation and column chromatography to obtain light yellow liquid Yield 74%; 1 H NMR (600MHz, CDCl3 )δ7.80(d, J=8.3Hz, 2H), 7.35(d, J=8.1Hz, 2H), 4.15(t, J=6.1Hz, 2H), 2.44(d, J=13.4Hz, 3H) ,2.26(dt,J=6.9,2.6Hz,2H),1.86(m,2H),1.23(m,1H);
[0073] 2) Will (35.7mg, 0.15mmol) was dispersed in 1mL acetonitrile, then added 18 F ions (100mCi), reacted at 80°C for 10min to obtain Yield 30%;
[0074] 3) Will (1g, 4.6mmol) was dispersed in 40mL of py...
Embodiment 3
[0079] A positron imaging agent 18 The preparation method of F-FPGalNAc comprises the following steps:
[0080] 1) Disperse 4-pentyn-1-ol (2.52g, 30mmol) in 100mL of dichloromethane, then add triethylamine (10.12g, 100mmol), 4-dimethylaminopyridine (0.16g, 1.32mmol) and p-toluenesulfonyl chloride (6.86g, 36mmol), react at room temperature for 20h, add ammonium chloride (5.35g, 0.1mol) to neutralize triethylamine, extract with ethyl acetate, and then remove the extract with anhydrous sodium sulfate Separated by water, vacuum distillation and column chromatography to obtain light yellow liquid Yield 81%; 1 H NMR (600MHz, CDCl 3 )δ7.80(d, J=8.3Hz, 2H), 7.35(d, J=8.1Hz, 2H), 4.15(t, J=6.1Hz, 2H), 2.44(d, J=13.4Hz, 3H) ,2.26(dt,J=6.9,2.6Hz,2H),1.86(m,2H),1.23(m,1H);
[0081] 2) Will (35.7mg, 0.15mmol) was dispersed in 1mL acetonitrile, then added 18 F ions (100mCi), reacted at 90°C for 10min to obtain Yield 35%;
[0082] 3) Will (1g, 4.6mmol) was dispersed in 20mL of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com