Pharmaceutical combination preparation and application thereof in preparation of medicine for treating aplastic anemia
An aplastic and pharmaceutical technology, applied in the application of pharmaceuticals, in the field of pharmaceutical combination preparations, can solve only 30-40% of the problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
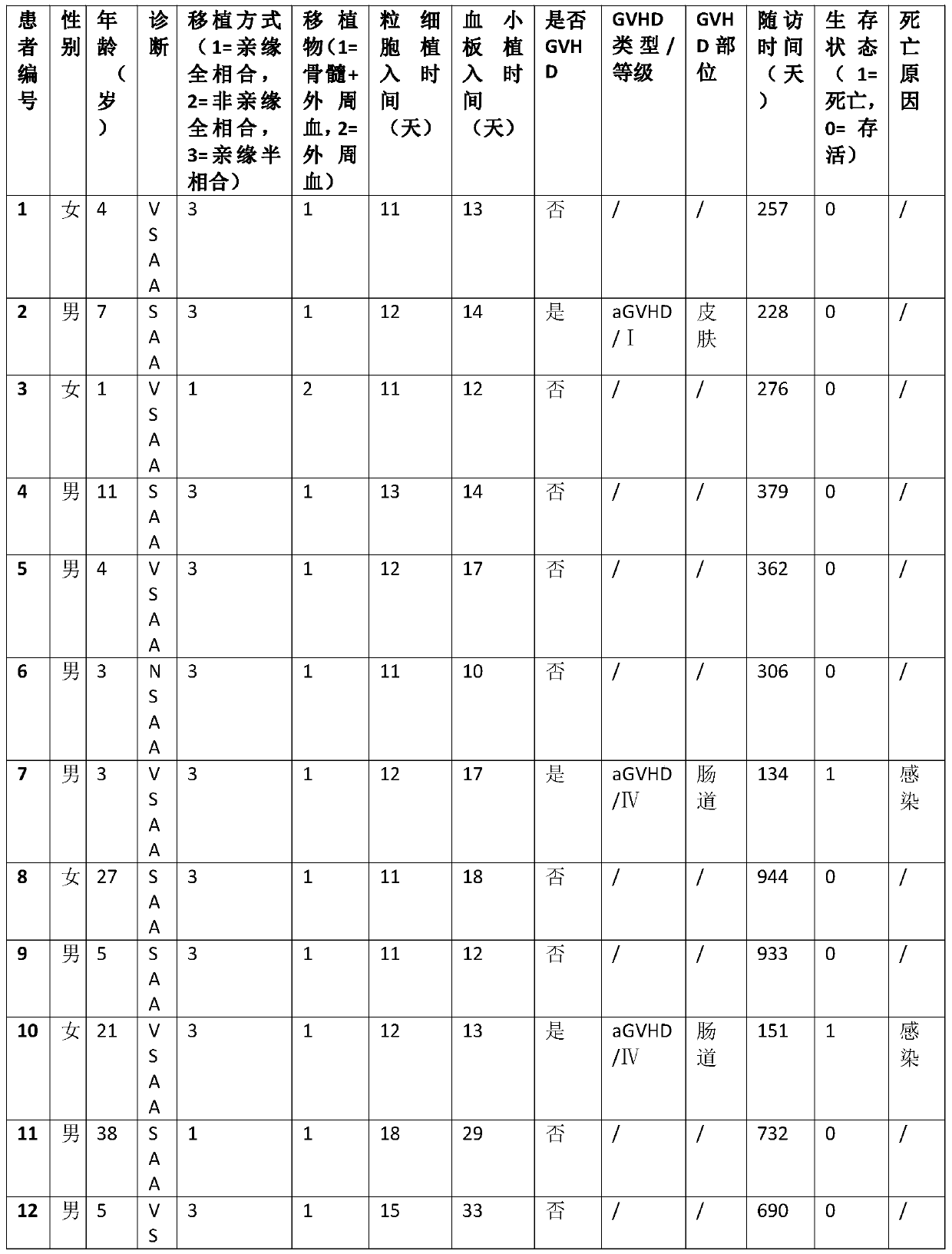
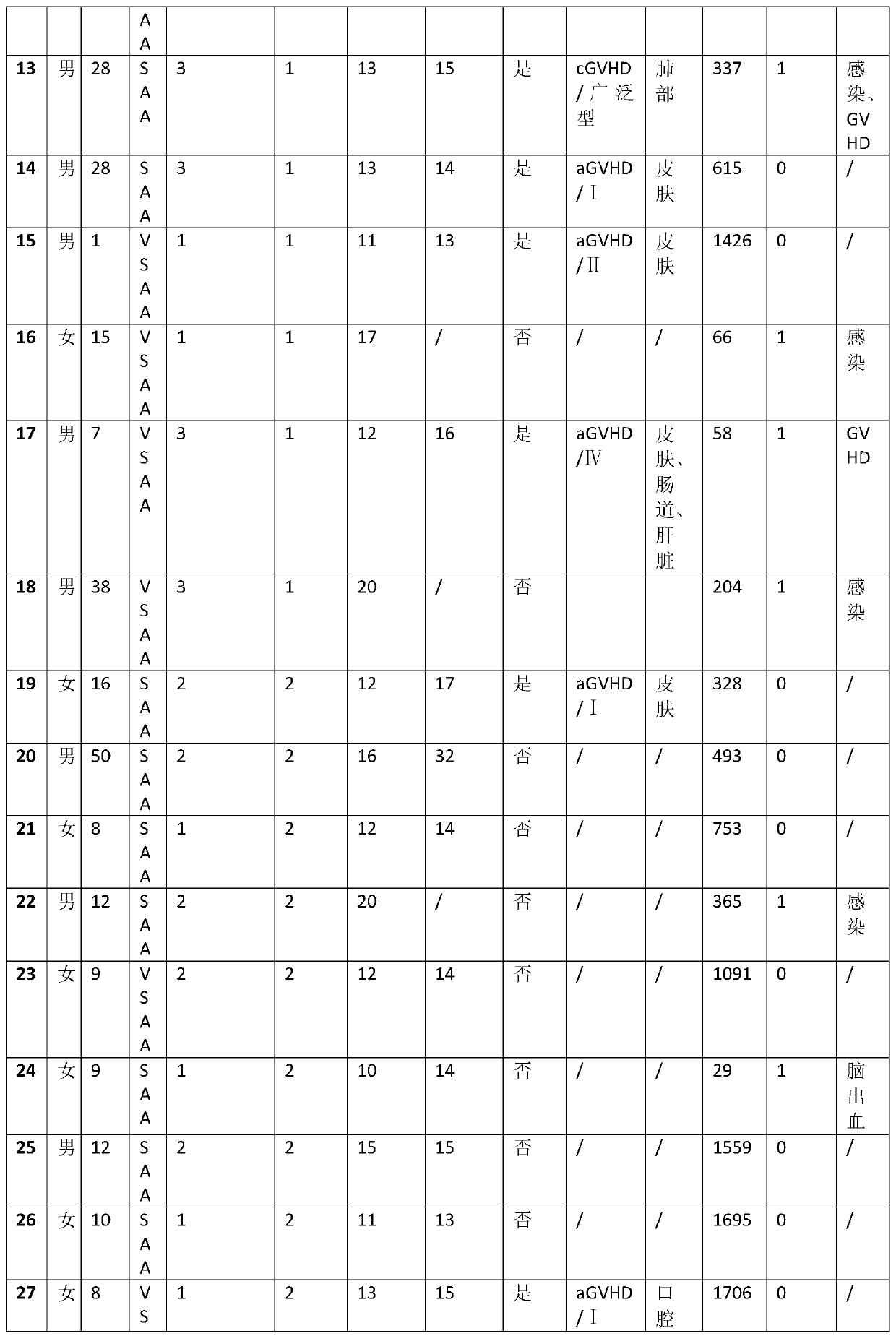
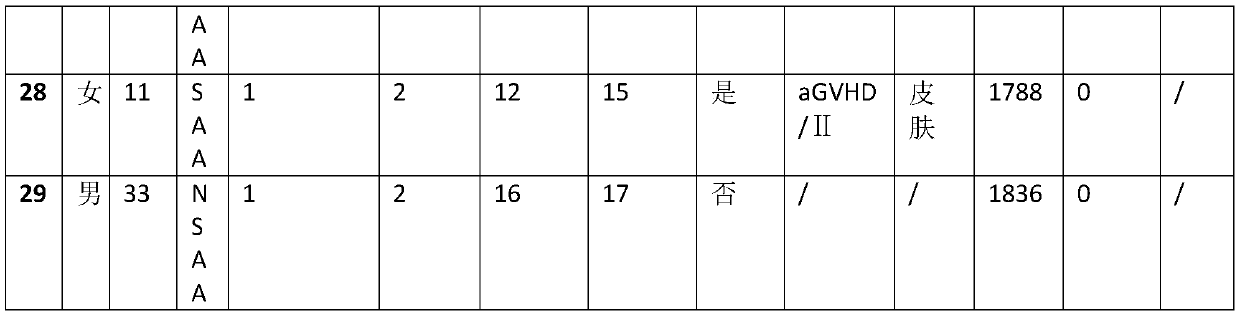
[0017] During 2013-2017, when 29 patients with aplastic anemia were treated with allogeneic hematopoietic stem cell transplantation, fludarabine 30mg / m was used 3 days before stem cell reinfusion treatment 2 / day fludarabine intravenous infusion for 4 days, at the same time 30mg / kg / day cyclophosphamide intravenous infusion for 4 days, and 10mg / kg / day anti-human T lymphocyte rabbit immune globulin intravenous infusion for 3 days at the same time, finally received Allogeneic hematopoietic stem cell transplantation. The 29 treated patients were followed up until October 2018, and the statistics of the follow-up results are as follows: Table 1:
[0018]
[0019]
[0020]
[0021] Table 1
[0022] VSAA in Table 1 is very severe aplastic anemia. SAA is severe aplastic anemia. NSAA is non-severe aplastic anemia. GVHD is Graft Versus Host Disease. aGVHD is acute graft-versus-host disease. cGVHD is chronic graft versus host disease.
[0023] According to the statistical...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com