5-pyrimidine-6-oxygen-pyrazolopyridine derivative and preparation method and application thereof
A technology of azolopyridines and derivatives, which is applied in the field of application as a drug for the treatment of pulmonary fibrosis, and can solve problems such as not having the ability to activate sGC
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
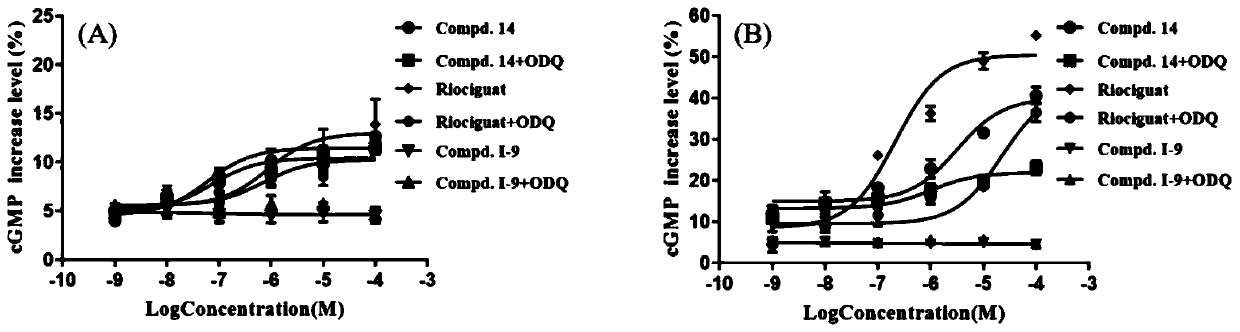
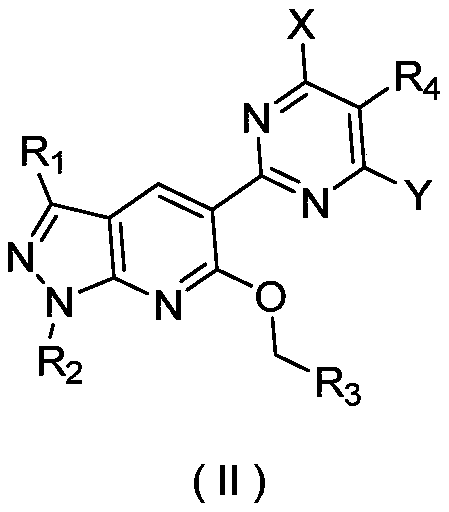
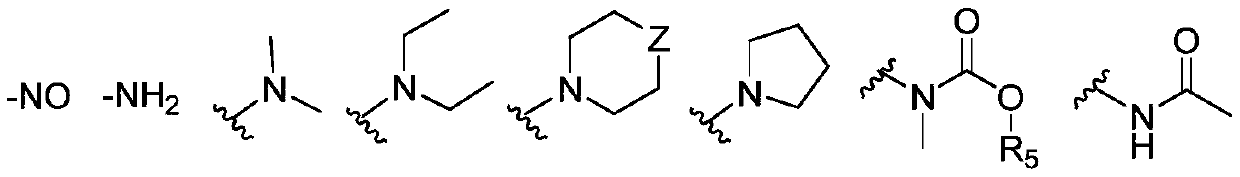
Image
Examples
Embodiment 1
[0084] 2-(6-((2-fluorobenzyloxy)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-5-yl)pyrimidine-4,6-di Phenol (compound 1)
[0085]
[0086] Weigh 0.22g of sodium methoxide (4mmol) into a 100mL eggplant-shaped bottle, then measure 40mL of methanol into the reaction flask, stir at room temperature, weigh 0.5g of compound 6-(2-fluorobenzyloxy)-3- Add methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carboxamidine (1.33mmol) into the reaction bottle, and weigh 0.18g of malonic acid with a plastic dropper by the reduction method Dimethyl ester (1.33 mmol) was slowly added dropwise into the reaction bottle, and finally 10 mL of methanol was added to rinse the bottle mouth. After transferring the reaction device to reflux at 70°C for 12 hours, TLC monitored the raw material 2-(6-((2-fluorobenzyloxy)-3-methyl-1-phenyl-1H-pyrazol[3,4- B] pyridine-5-pyridyl) formamidine is not completely reacted. TLC monitors raw material 2-(6-((2-fluorobenzyloxy)-3-methyl-1-phenyl-1H-pyridine after conti...
Embodiment 2
[0088] 2-(6-((2-fluorobenzyloxy)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-5-yl)-5-morpholinopyrimidine -4,6-diphenol (compound 2)
[0089]
[0090] According to the synthetic method of compound 1, the compound 6-(2-fluorobenzyloxy)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carboxamidine and morphine Diethyl linylmalonate was used as starting material to obtain a yellow solid. Yield: 34%, melting point: 221.7-221.9°C, HPLC: 96.98%. 1 H NMR (400MHz, DMSO-d 6 ):δ12.03(br s,1H,-OH),8.63(s,1H),8.12(d,J=7.7Hz,2H,Ar-H),7.65~7.57(m,1H,Ar-H) ,7.54(t,J=8.0Hz,2H,Ar-H),7.41(m,1H,Ar-H),7.32(m,1H,Ar-H),7.21(t,J=7.4Hz,1H, Ar-H),5.66(s,2H,-CH 2 -),3.71~3.65(m,4H,-CH 2 -),3.09~3.02(m,4H,-CH 2 -),2.58(s,3H,-CH 3 ). 13 C NMR (101MHz, DMSO-d 6 ):δ160.44(d, 1 J C,F =244.0Hz),160.06,151.65(2C),148.57,144.80,139.12,135.22,130.59,130.51,130.40,129.68(2C),126.16,124.97,124.07(d, 2 J C,F =14.0Hz), 120.14(2C), 115.83(d, 2 J C,F =21.0Hz),113.65,112.48,112.35,66.91(2C),63.18,49....
Embodiment 3
[0092] 2-(6-((2-fluorobenzyloxy)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridin-5-yl)-5-piperidinylpyrimidine -4,6-Diphenol (Compound 3)
[0093]
[0094] According to the synthetic method of compound 1, the compound 6-(2-fluorobenzyloxy)-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carboxamidine and piperidine Diethyl pyridyl malonate was used as starting material to obtain a white solid. Yield: 41%, melting point: 230.0-231.5°C, HPLC: 97.34%. 1 H NMR (400MHz, DMSO-d 6 ):δ11.38(br s,1H,-OH),8.62(s,1H),8.17~8.07(d,J=7.8Hz,2H,Ar-H),7.66~7.58(m,1H,Ar- H), 7.53(t, J=7.9Hz, 2H, Ar-H), 7.44~7.35(m, 1H, Ar-H), 7.34~7.26(dd, J=16.0, 8.1Hz, 2H, Ar-H ), 7.20(t, J=7.4Hz, 1H, Ar-H), 5.66(s, 2H, -CH 2 -),3.34(m,4H,-CH 2 -),2.58(s,3H,-CH 3 ),1.76(m,4H,-CH 2 -),1.50(m,2H,-CH 2 -). 13 C NMR (101MHz, DMSO-d 6 ):δ160.44(d, 1 J C,F =244.0Hz),160.04,152.76(2C),148.51,144.85,139.05,135.14,130.58,130.50,130.32,129.64(2C),126.15,124.94,124.03(d, 2 J C,F =14.0Hz), 120.11(2C), 115.79...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com