Multifunctional crosslinked polyarylidene butanedione anion exchange membrane and preparation method thereof
A poly(arylene butanedione), anion exchange membrane technology, applied in electrochemical generators, fuel cells, electrical components, etc. performance, good alkali resistance and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
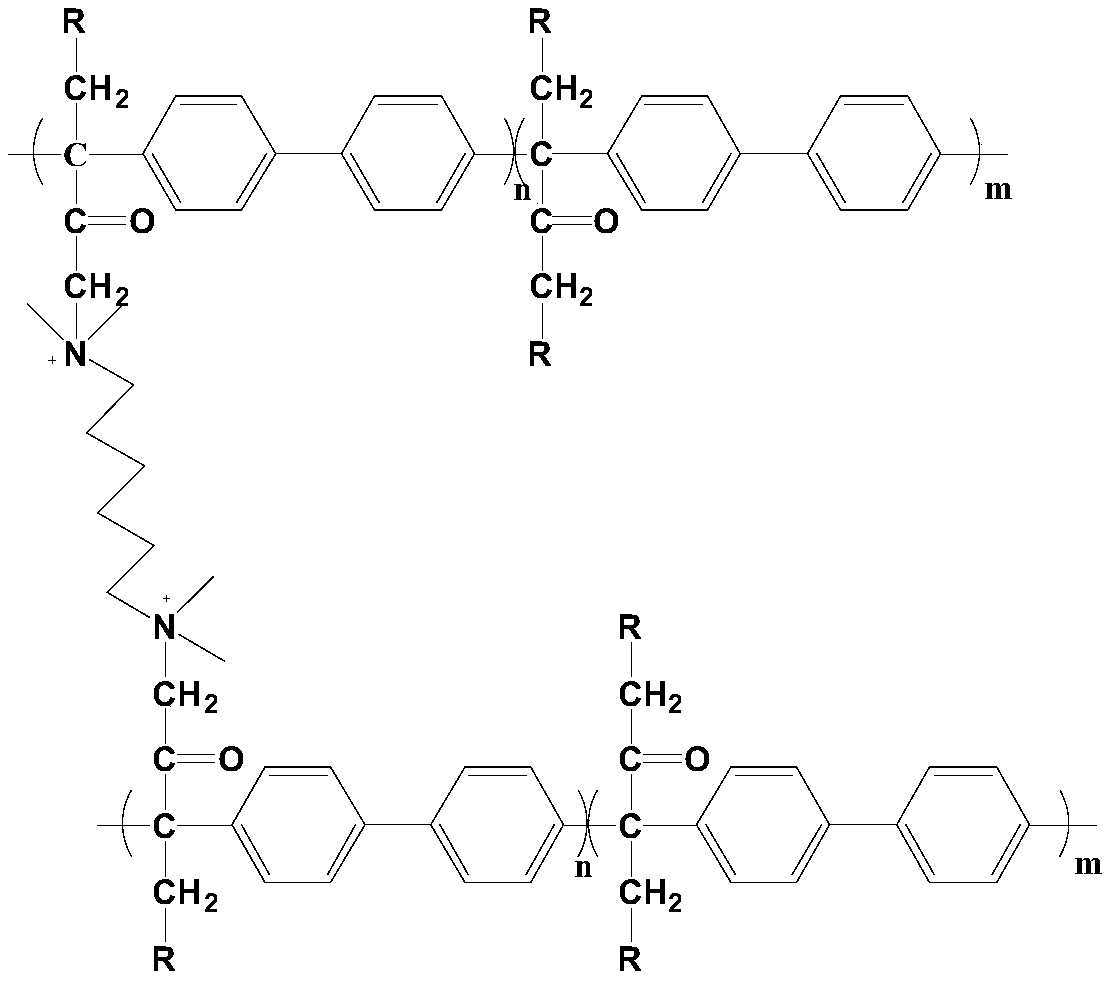
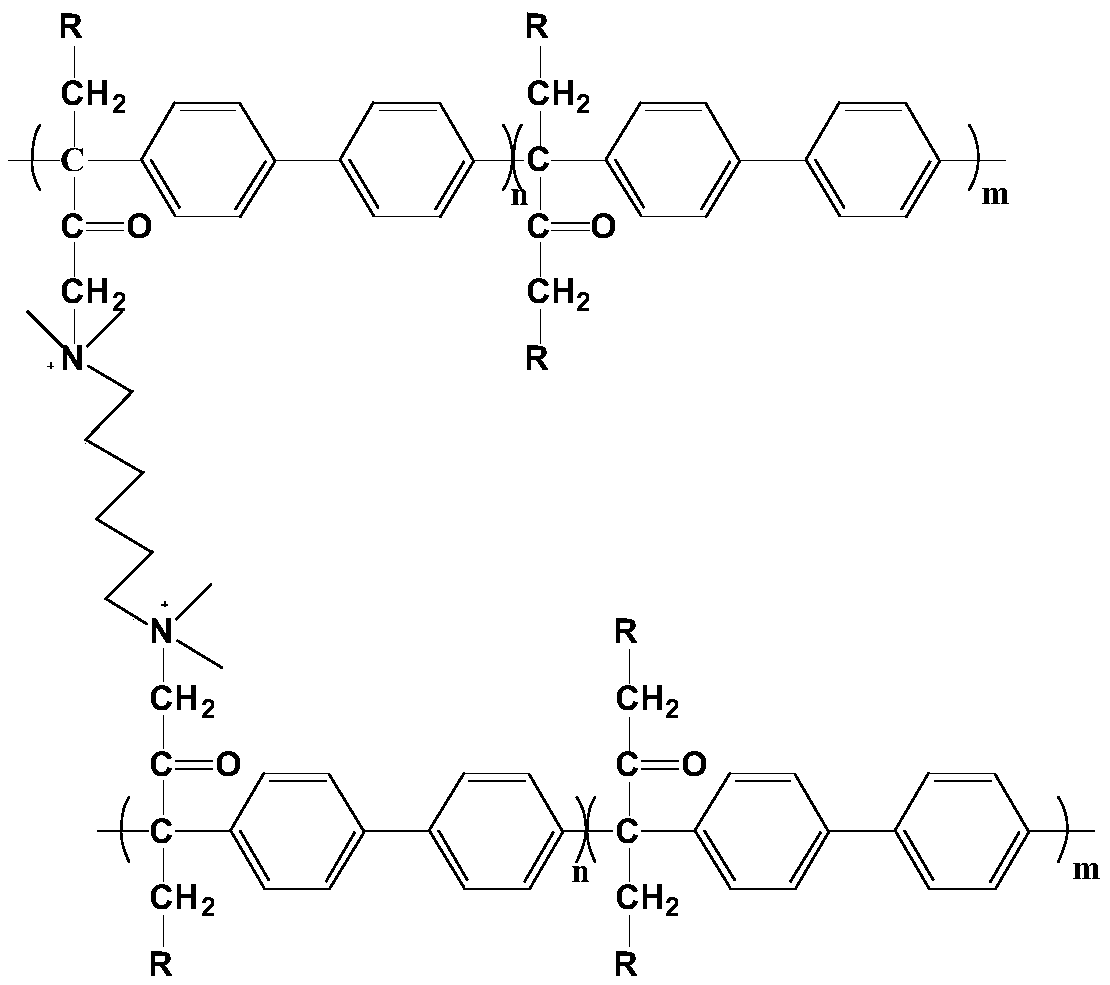
[0030] Synthesis of dibromo-substituted polyarylene butanedione: Add 4.024g (16.5mmol) of 1,4-dibromo-2,3-butanedione and 2.313g (15mmol) of biphenyl into a 50mL single-necked flask, Add 22.5mL of dichloromethane and 5.3mL of trifluoromethanesulfonic acid, react at 0°C for 6h. After the reaction is completed, the product is slowly poured into methanol under stirring, and a filamentous polymer product is precipitated. The product is repeatedly washed and soaked with methanol, and then dried to obtain a polymer. Dissolve the polymer with N-methylpyrrolidone into a solution of a certain concentration. After the solution is completely dissolved, pour the solution into methanol to precipitate the polymer. After repeated washing several times, dry it in a vacuum oven for 6 hours at room temperature to obtain a polymer product.
[0031] Synthesis of combined polyarylene butanedione anion exchange membrane: Dissolve 0.16 g of dibromosubstituted polyarylene butanedione in 4.4 mL of N-m...
Embodiment 2
[0037] Synthesis of dibromo-substituted polyarylene butanedione: Add 4.024g (16.5mmol) of 1,4-dibromo-2,3-butanedione and 2.313g (15mmol) of biphenyl into a 50mL single-necked flask, Add 22.5mL of dichloromethane and 5.3mL of trifluoromethanesulfonic acid, react at 0°C for 6h. After the reaction is completed, the product is slowly poured into methanol under stirring, and a filamentous polymer product is precipitated. The product is repeatedly washed and soaked with methanol, and then dried to obtain a polymer. Dissolve the polymer with N-methylpyrrolidone into a solution of a certain concentration. After the solution is completely dissolved, pour the solution into methanol to precipitate the polymer. After repeated washing several times, dry it in a vacuum oven for 6 hours at room temperature to obtain a polymer product.
[0038] Synthesis of combined polyarylene butanedione anion exchange membrane: Dissolve 0.16 g of dibromosubstituted polyarylene butanedione in 4.4 mL of N-m...
Embodiment 3
[0044] Synthesis of dibromosubstituted polyarylene butanedione: same as Example 2
[0045]Synthesis of combined polyarylene butanedione anion exchange membrane: Dissolve 0.16 g of dibromosubstituted polyarylene butanedione in 4.4 mL of N-methylpyrrolidone in a 25 mL one-necked flask, add 4.4 μL of N,N, N',N'-tetramethyl-1,6-hexanediamine, stirred for 30 minutes, centrifuged the casting solution and cast it in a glass mold, dried at 30°C for 24 hours, and then heated to 60°C for 24 hours to obtain a polymer film .
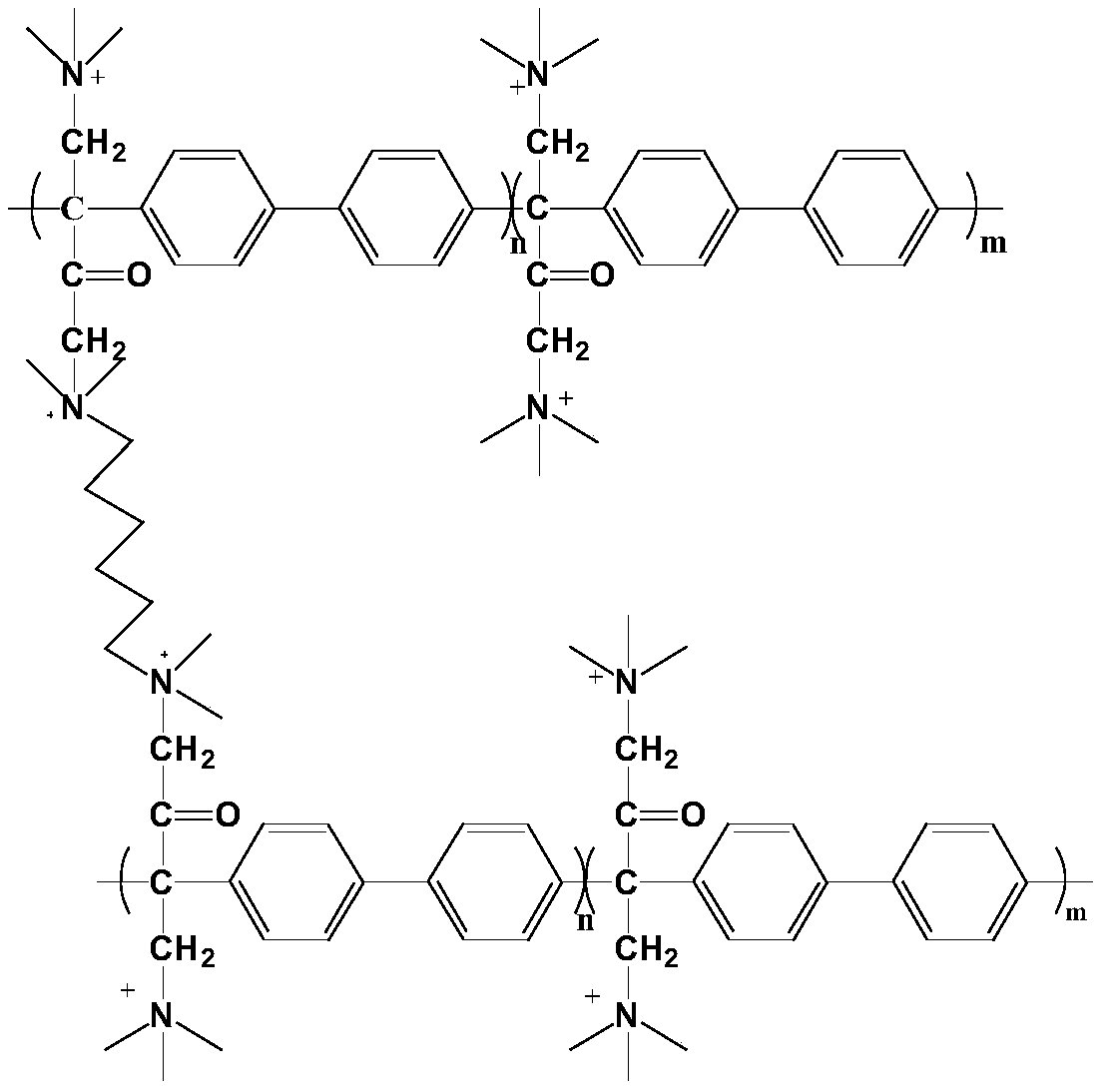
[0046] Preparation of multifunctional cross-linked polyarylene butanedione anion exchange membrane: Soak the joint polyarylene butanedione anion exchange membrane in 10% N-methylpiperidine aqueous solution at 80°C for 48h, Then soak in deionized water for 24 hours, then soak in 1mol / L KOH solution at room temperature for 48 hours, then repeatedly wash with deionized water and soak for 48 hours until neutral, then the multi-functional cross-linked polyarylene Diace...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com