Pancreatic cancer specific binding peptide, preparation method and use thereof
A specific binding, pancreatic cancer technology, applied in the biological field, can solve the problems of large individual differences in therapeutic effects, high manufacturing costs, and high production costs, and achieve the effects of simple synthesis and purification, good effect, and weak immunogenicity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 Three rounds of subtractive screening of pancreatic cancer cell specific binding positive polypeptides using phage display technology
[0054] 1.1 Recovery and cultivation of host bacteria E.coli ER2738
[0055] To prepare an E. coli plate, take the LB-TET culture plate and preheat it in a 37-degree incubator for 1 hour. After the E.coli ER2738 bacterial liquid melts, use an inoculation loop to dip a small amount of bacterial liquid evenly on the culture plate, and then place it upside down at 37°C overnight in a constant temperature incubator. Prepare the host bacterial solution, pick a single colony from a well-grown culture plate, place it in LB bacterial culture solution containing tetracycline, and cultivate overnight at 37°C with shaking at 180rpm, so that the bacteria are in the logarithmic growth phase. The prepared LB-Tet plate containing Escherichia coli was stored in a 4°C refrigerator for later use, and the host bacterial solution was stored in a ...
Embodiment 2
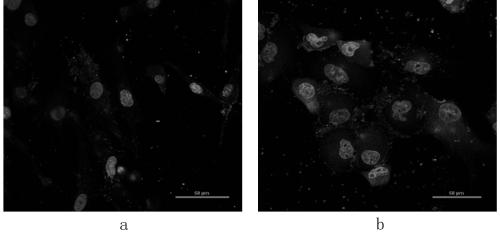
[0079] Example 2 Determination and Analysis of DNA Sequence of Positive Phage Clones and Cell Immunohistochemical Identification of Targeted Binding Ability of Positive Phage Clones to Pancreatic Cancer Cells
[0080] 2.1 Determination and analysis of positive phage clone DNA sequence
[0081] 2.1.1 Positive monoclonal phage selection
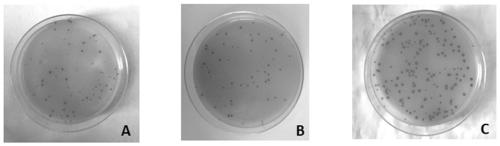
[0082] The phage liquid obtained after the third round of screening was titered and spread on LB plates, and on the plates with less than 100 growing spots, 44 well-growing blue spots at an interval of 5 mm were randomly selected. Add 44 randomly picked coeruleus into 1 ml pre-logarithmic host bacterial solution (same as phage amplification), and amplify at 37°C and 200 rpm for 4.5 hours with rapid shaking.
[0083] 2.1.2 Positive monoclonal phage single-stranded DNA extraction
[0084] Centrifuge the amplified monoclonal phage liquid at 4°C and 14000rpm for 30 seconds, transfer the supernatant to a new tube, centrifuge at 4°C and 1000rpm for...
Embodiment 3
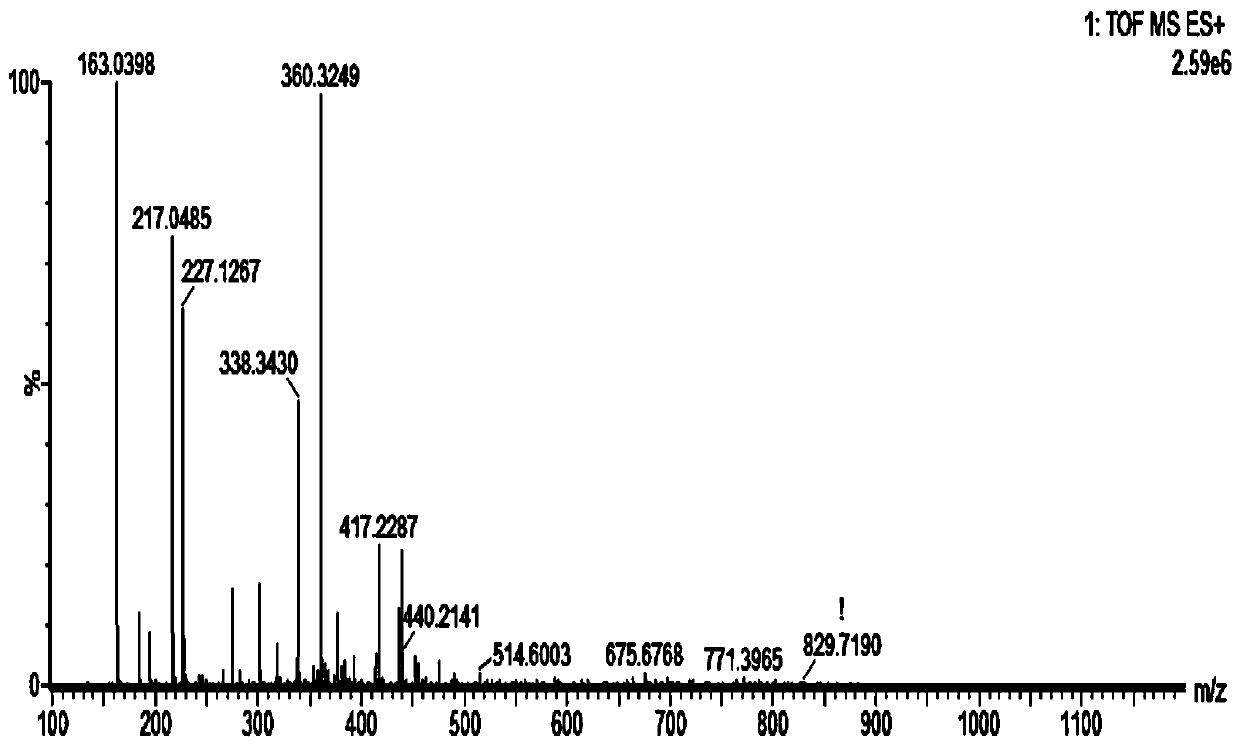
[0094] Example 3 Solid-phase synthesis of polypeptides and FITC-positive polypeptide fragments thereof
[0095] According to the measured amino acid sequence, the homology comparison analysis of its amino acid sequence and the bioinformatics analysis of its nucleic acid sequence are carried out. Polypeptide FY-YR (sequence is FYSHSNLNLKYR), FH-YN (sequence is FHLYLRYNLKYN), AD-YR (sequence is ADARYKSNLKYR), FH-YR (sequence is FHLFFLCRGDYR), DY-YN (sequence is DYHDPNLPTDYN ) by solid phase synthesis ) and FITC-positive polypeptide fragments FITC-FY-YR (sequence is FYSHSNLNLKYR), FITC-FH-YN (sequence is FHLYLRYNLKYN), FITC-AD-YR (sequence is ADARYKSNLKYR), FITC-FH-YR (sequence is FHLFFLCRGDYR) , FITC-DY-YN (sequence is DYHDPNLPTDYN), and identify the synthesized polypeptide. At the same time, the above polypeptides and FITC-positive polypeptide fragments were synthesized at Sangon Bioengineering (Shanghai) Co., Ltd.
[0096] 3.1 Preparation method of polypeptide FY-YR
[0097...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com