Nucleic acid aptamer and its circular bivalent nucleic acid aptamer-drug coupling system and application thereof
A nucleic acid aptamer, nucleic acid aptamer technology, applied in the field of biomedicine, can solve the problem of less nucleic acid aptamer, and achieve the effects of strong affinity, good affinity, and reduced damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 azide-drug
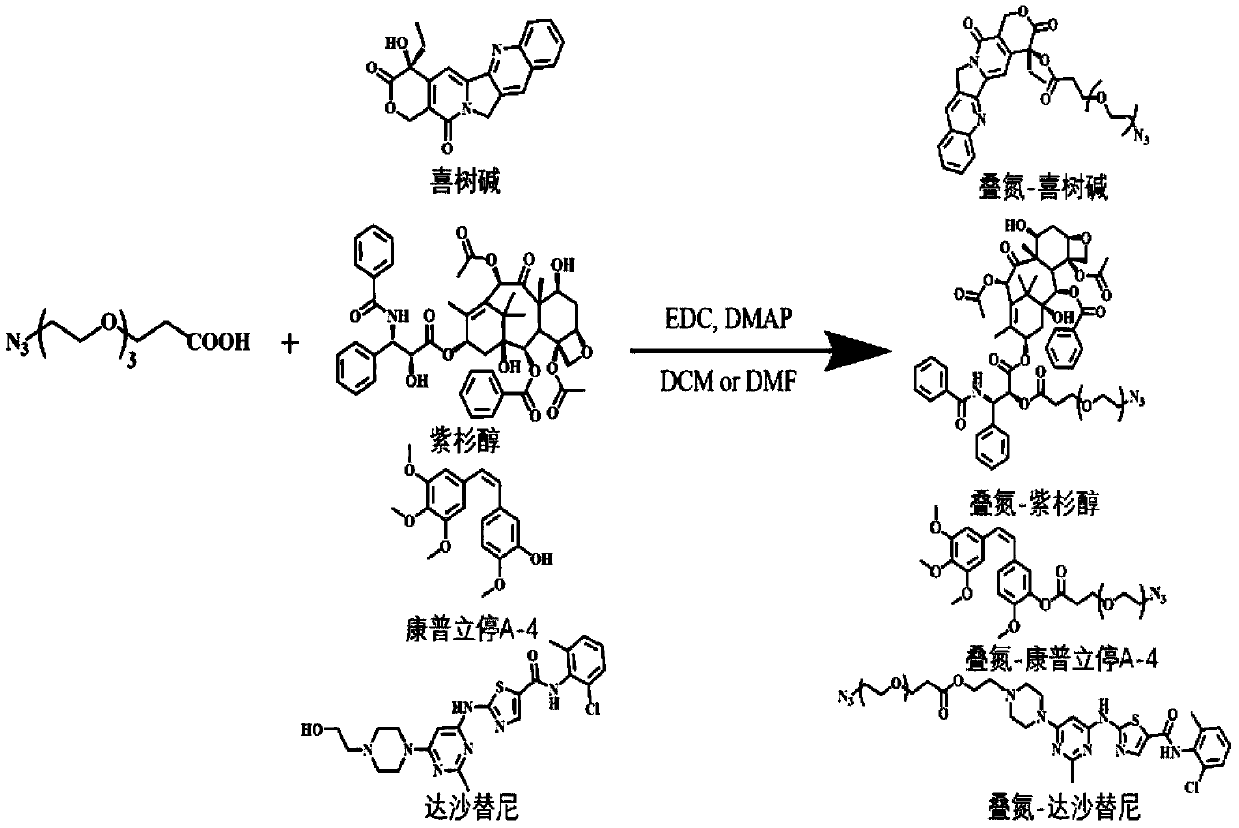
[0034] The synthetic route of described azide-drug, such as figure 1 Shown, specifically include the synthesis of the following azide-drugs.
[0035] Synthesis of azide-camptothecin
[0036] Camptothecin (64mg, 0.18mmol), azide-PEG 3 -Carboxyl (86mg, 0.37mmol), N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (EDC·HCl, 71mg, 0.37mmol) and 4-dimethyl A solution of a mixture of aminopyridines (DMAP, 45 mg, 0.37 mmol) in DCM (10 mL) at room temperature under N 2 Stir overnight. The reaction mixture was then concentrated in vacuo and the product was purified by flash chromatography to give a yellow solid yielding azide-camptothecin (CPT).
[0037] Synthesis of Azide-paclitaxel
[0038] Paclitaxel (86mg), azide-PEG 3 -Carboxyl (25mg), a mixture of N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (EDC·HCl, 38mg) and 4-dimethylaminopyridine (DMAP , 24 mg, 0.37 mmol) in DCM (10 mL) at room temperature un...
Embodiment 2
[0043] The modification of embodiment 2 nucleic acid aptamers
[0044] The nucleic acid sequence of the aptamer shown in SEQ ID NO: 2 is modified by a phase synthesizer with a dibenzocyclooctyne functional group (DBCO), and the modified sequence XQ-2d-1 is as follows: TGACTGATTTACGGCTCATAG GGT( DBCO) TAGGGGCTGCTGGCCAGA TAC TCA GATGGTAGG GTTACTATGAGC.
[0045] The nucleic acid sequence of the aptamer shown in SEQ ID NO: 3 is modified by a phase synthesizer with two dibenzocyclooctyne functional groups (DBCO), and the modified sequence XQ-2d-2 is as follows: CGTAAATCAGTCAGCATAGGGT (DBCO )TAGGGGCTGCT(DBCO)GGCCAGATACTCAGATGGTAGGGTT ACTATGAGC
[0046] The nucleic acid sequence of the aptamer shown in SEQ ID NO: 2 is modified by a phase synthesizer with three dibenzocyclooctyne functional groups (DBCO), and the modified sequence XQ-2d-3 is as follows: TGACTGATTT (DBCO )ACGGCTCATAGGGT(DBCO)TAGGGGCTGCT(DBCO)GGCCAGATACTCAGATGGTAGGGTTACTATGAGC.
Embodiment 4
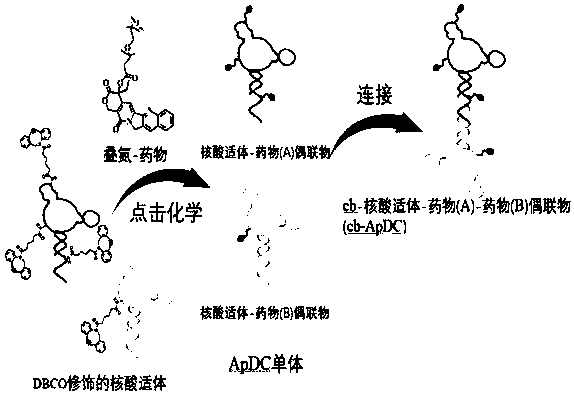
[0047] Example 4 Preparation of Nucleic Aptamer-Drug Conjugate Monomer (ApDC)
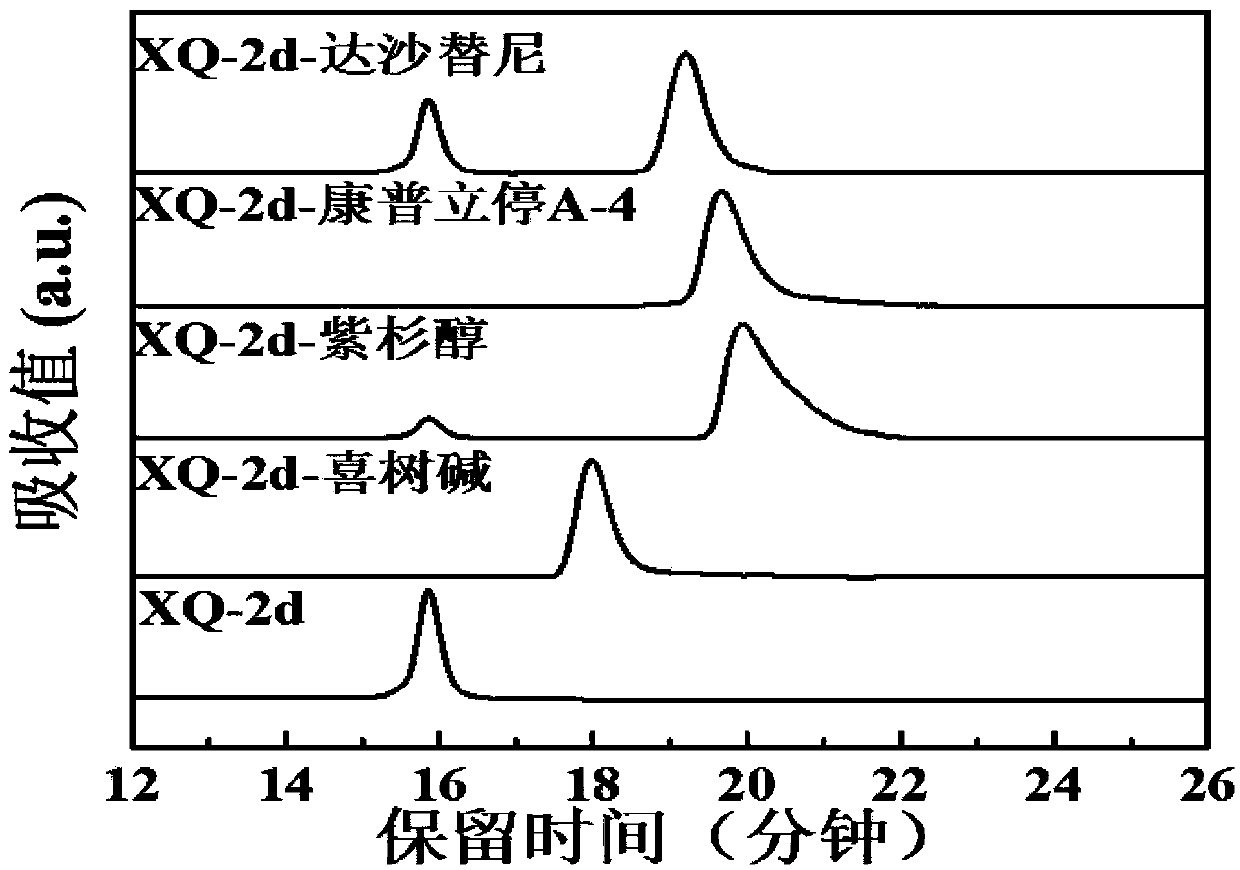
[0048] Preparation of XQ-2d-CPT: the following reaction system was prepared in Duchenne's phosphate buffer: nucleic acid aptamer XQ-2d-1 (Example 2) 1OD, azide-camptothecin (Example 1) 1mM, two The volume percentage of methyl sulfoxide is 10%. The above reaction system was placed at 37°C and reacted for 8h. The product was purified by HPLC and then lyophilized and concentrated to obtain the monomer XQ-2d-CPT. The resulting solution was stored at -20°C until use.
[0049] Preparation of XQ-2d-2CPT: The preparation method is the same as XQ-2d-CPT, only XQ-2d-1 is replaced by XQ-2d-2.
[0050] Preparation of XQ-2d-3CPT: The preparation method is the same as that of XQ-2d-CPT, only XQ-2d-1 is replaced by XQ-2d-3.
[0051] Preparation of XQ-2d-PTX: The preparation method is the same as that of XQ-2d-CPT, except that azide-camptothecin is replaced by azide-paclitaxel.
[0052] Preparation of XQ-2d-2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com