Determining method for drug loading in preparation process of solid dispersion
A technology of solid dispersion and determination method, which is applied in the field of biomedicine to achieve the effect of cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
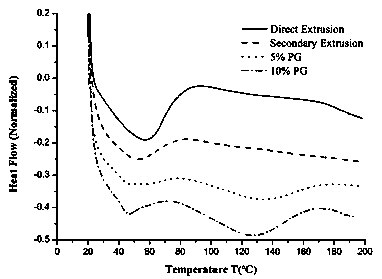
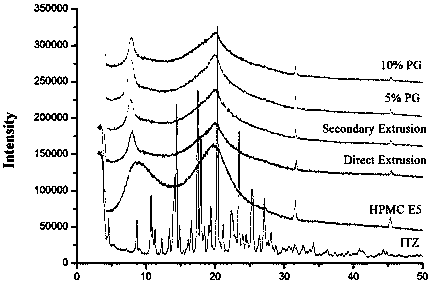
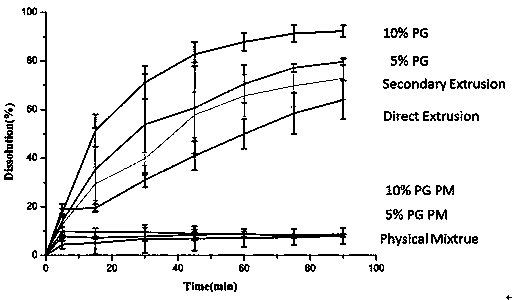
[0034] Preparation of Itraconazole Solid Dispersion and Selection of Excipients
[0035] Test materials and equipment
[0036] Itraconazole (batch number: A-10511611011, Shouguang Fukang Pharmaceutical); hydroxypropyl methylcellulose (batch number: PD441808, Dow Chemical); propylene glycol (PG, Guangzhou Meiyi Biotechnology Co., Ltd.); hot melt extrusion machine (Hake Minilab, Thermo Fisher Co., Ltd.); thermogravimetric analyzer (Discovery TGA, Waters Corporation, USA); differential scanning calorimeter (Discovery DSC, Waters Corporation, USA); intelligent dissolution apparatus (Distek 7100, Xinghe Instrument Co., Ltd.); automatic sampler (Distek 4300, Xinghe Instrument Co., Ltd.); ultraviolet spectrophotometer (Distek S-3100, Xinghe Instrument Co., Ltd.); X-ray powder diffractometer (D8 Advance , American Bruker Dalton Company); other reagents were of analytical grade.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com