Method for synthesizing doxorubicin-coupled targeting polypeptide
A technology for targeting peptides and synthesis methods, applied in the preparation methods of peptides, chemical instruments and methods, peptides, etc., can solve the problems of unclear target products, many side reactions, and complex final products, and reduce the formation of by-products. Opportunities, Yield Improvement, Effect of Increased Bonding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
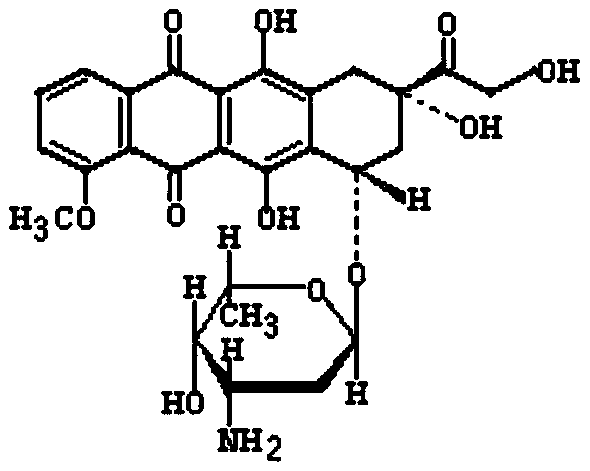
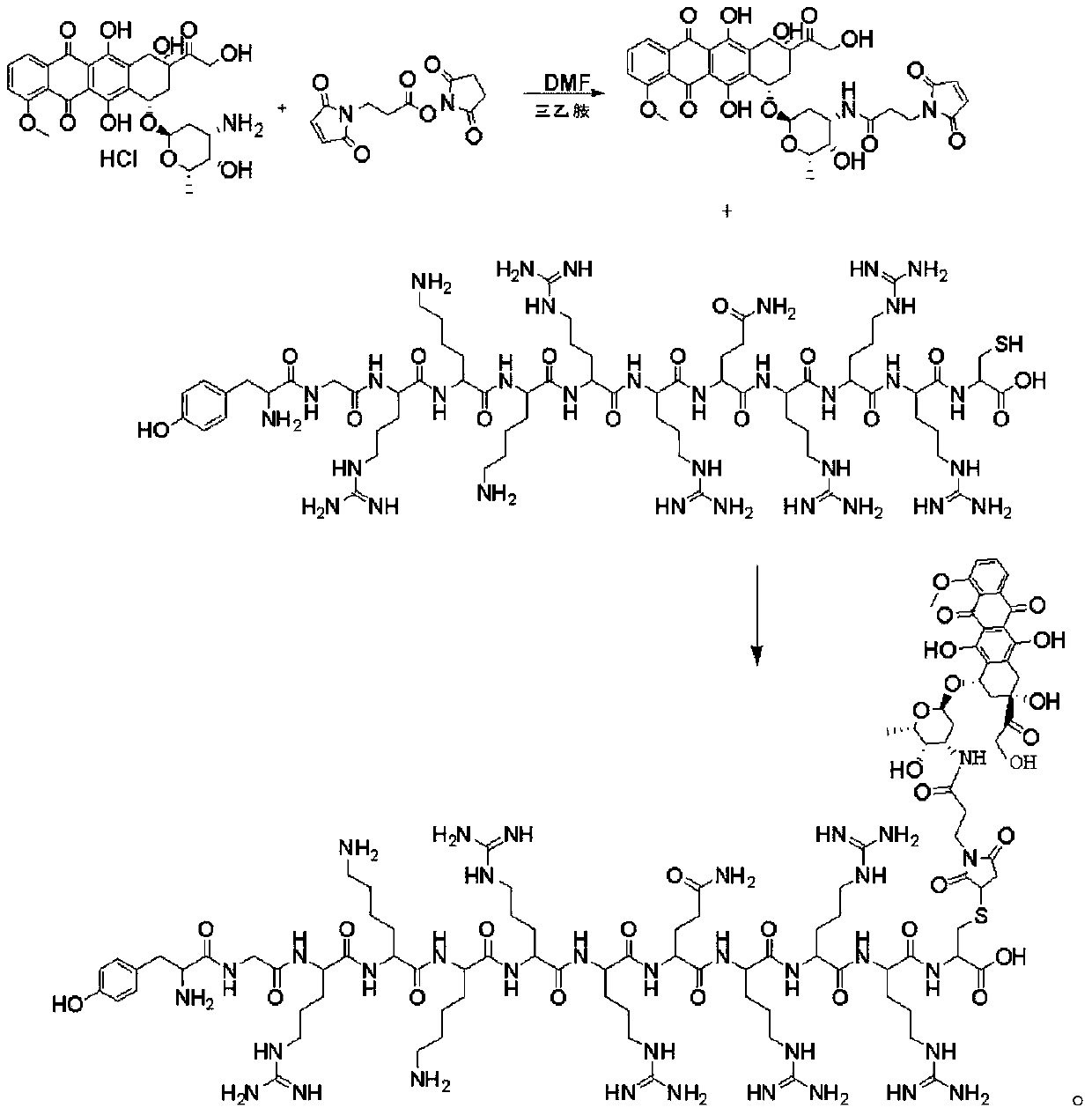
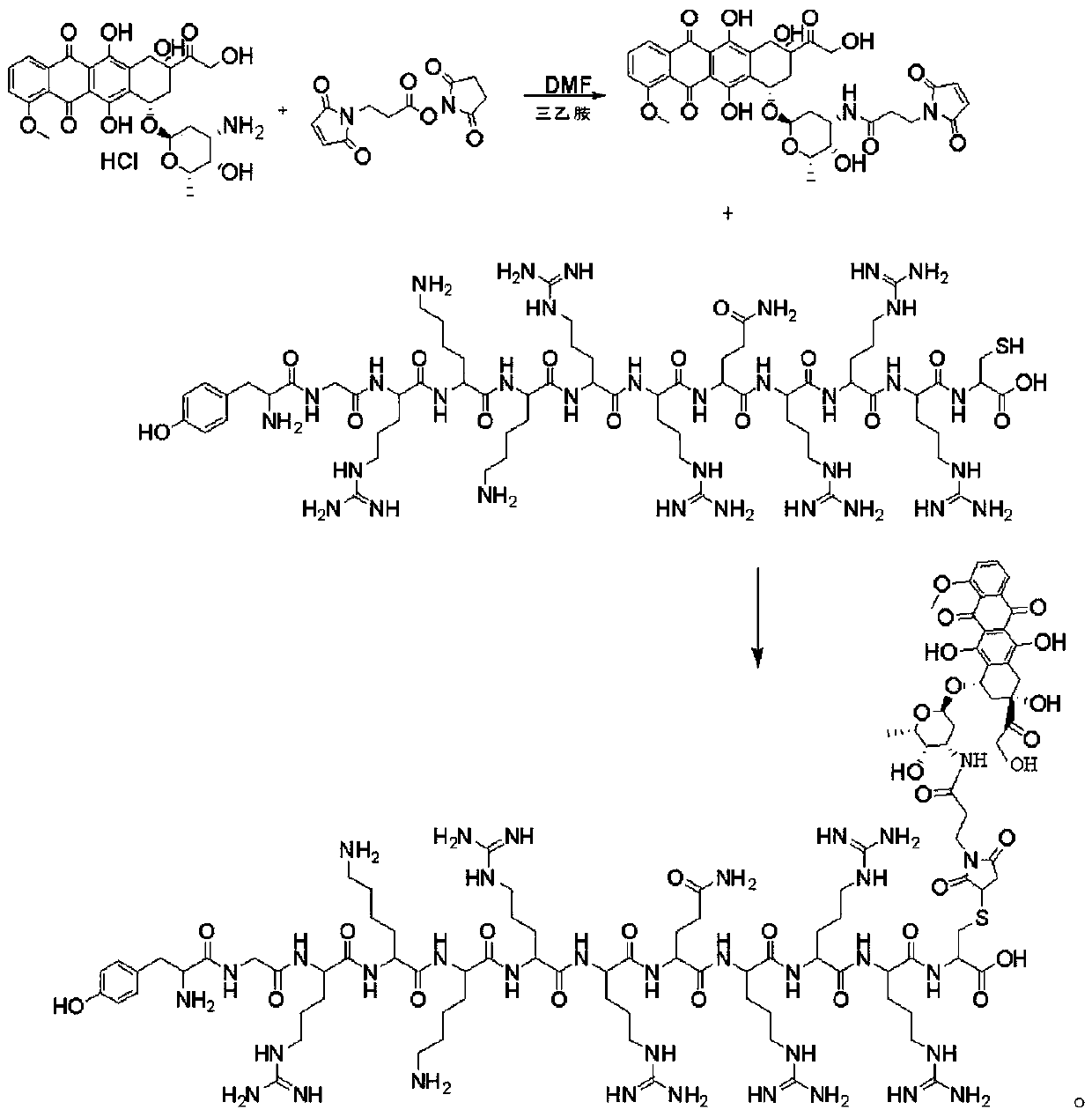
[0063] The invention discloses a method for synthesizing doxorubicin-coupled targeting polypeptide, which comprises the following steps:
[0064] S1, Preparation of Resin Peptide NH 2 -A 1 -A 2 -...-A n -Cys(Trt)-Resin;
[0065] Cys(Trt), A n , ... and A 1 Link to resin Resin in turn;
[0066] Among them, A n Is the amino acid -Ala-, -Met-, -Phe-, -Pro-, -Gly-, -Ile-, -Leu-, -Val-, -Arg(Pbf)-, -Lys(Boc)-, -Trp (Boc)-, -Ser(tBu)-, -Thr(tBu)-, -Tyr(tBu)-, -Asp(OtBu)-, -Glu(OtBu)-, -Asn(Trt)- or -Gln( Trt)-, n is a natural number not less than 1.
[0067] S2, preparation of polypeptide fragment NH 2 -A 1 -A 2 -...-A n -Cys-COOH;
[0068] NH 2 -A 1 -A 2 -...-A n -Resin and side chain protecting groups on Cys(Trt)-Resin are removed, purified to obtain NH 2 -A 1 -A 2 -...-A n - Pure Cys-COOH.
[0069] S3, preparing the intermediate doxorubicin-Mal;
[0070] The intermediate doxorubicin-Mal was prepared by using doxorubicin hydrochloride and BMPS.
[0071] S4...
Embodiment 2
[0095] S1, Preparation of Resin Peptides
[0096] NH 2 -Tyr(tBu)-Gly-Arg(Pbf)-Lys(Boc)-Lys(Boc)-Arg(Pbf)-Arg(Pbf)-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-Arg (Pbf)-Cys(Trt)-2-Chlorotrityl Chloride resin;
[0097] a. Add 1g of 0.50mmol / g 2-Chlorotrityl Chloride resin to the reaction vessel, and add DCM, blow the 2-Chlorotrityl Chloride resin with nitrogen for 15 minutes to fully swell the 2-ChlorotritylChloride resin, and then pump it through the sand core Filter off the solvent;
[0098] b. Add 1.5mmol of protected amino acid Fmoc-Cys(Trt)-OH into the reaction vessel, first add DCM to dissolve, then add 10eq of DIEA, react for 60min, and block with methanol during the reaction to obtain Fmoc-Cys(Trt) -Resin;
[0099] c. Remove Fmoc group: Add 10ml of 20% piperidine / DMF solution to the reactor, stir the reaction for 5min under nitrogen protection, and remove the liquid; wash with 10ml DMF twice, then add 10ml eluent, and stir the reaction Extract after 10 minutes; wash with 10ml DMF, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com