Expression method of AEP cyclase in pichia pastoris and application thereof
A Pichia pastoris, expression method technology, applied in the field of genetic engineering, can solve the problem that the expression amount cannot meet industrial production and the like, and achieve the effects of improving stability and prolonging half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Expression of AEP cyclase in Pichia pastoris
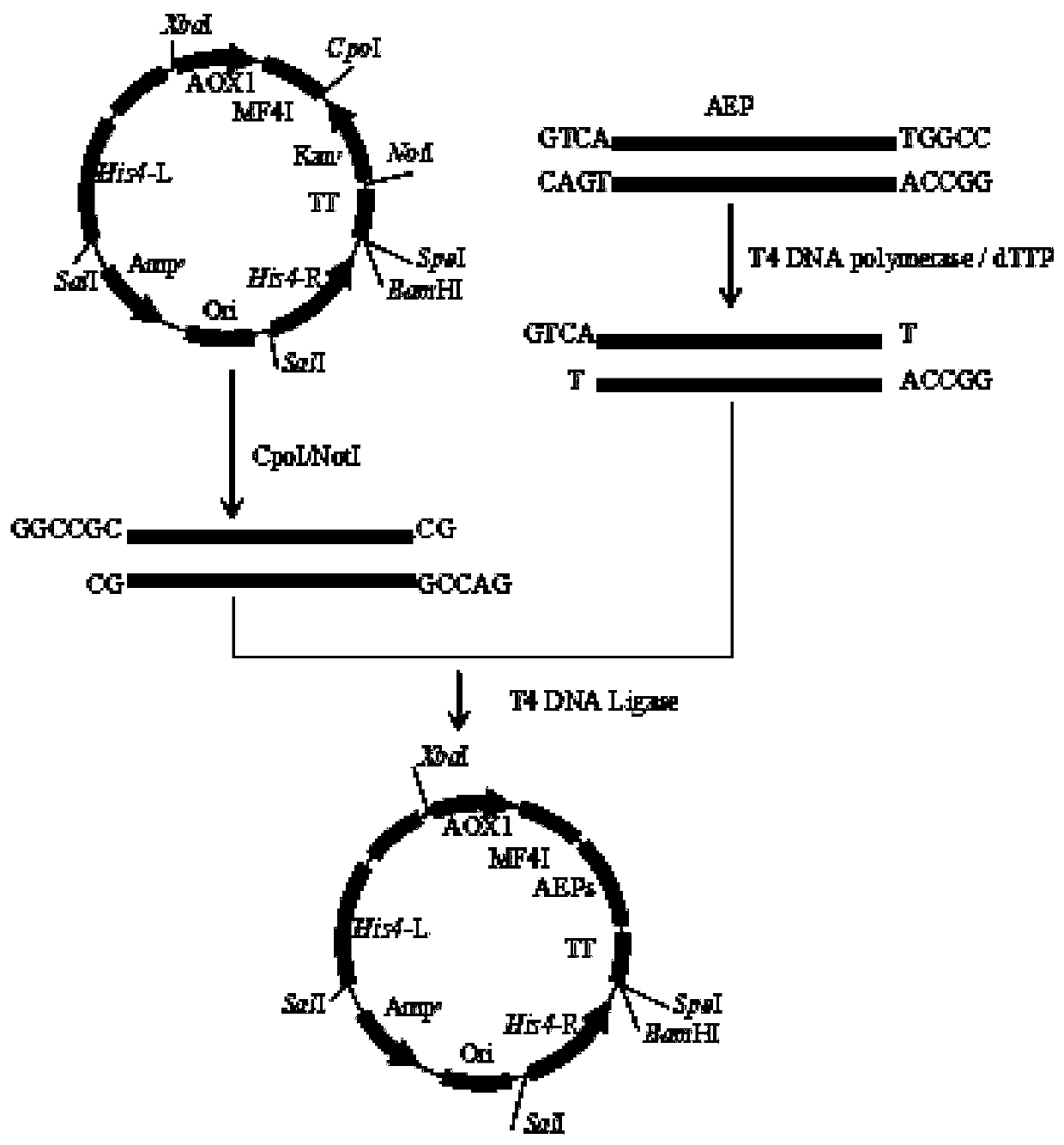
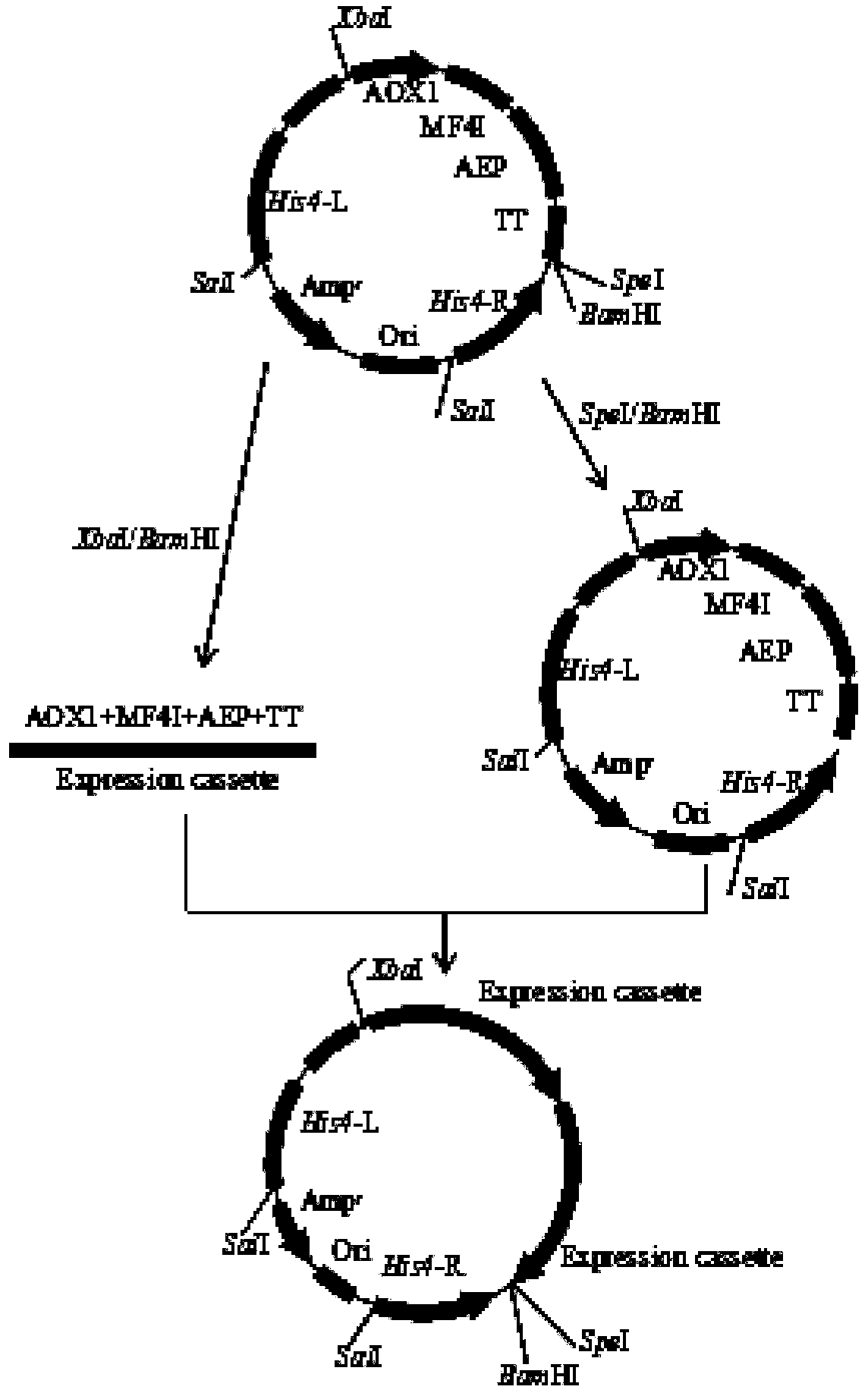
[0039] S1: Construction of pBDM-AEP vector
[0040] Construction principle see figure 1 .
[0041] First, the amino acid sequence of AEP was obtained from the literature, and the sequence was handed over to Wuhan Jinkairui Bioengineering Co., Ltd. for gene synthesis. Using the plasmid containing the AEP sequence provided by Wuhan Jinkairui Bioengineering Co., Ltd. as a template, BDM-AEP-F: GTCAGCACGTGATGGTGATTATCTTCATTTACCG, see SEQ ID NO: 1 and BDM-AEP-R: GGCCATTAAGGAATAGAAGCGCATGCCTGAGAGG, see SEQ ID NO: 2, for Forward and reverse primers for PCR amplification of AEP fragments, the total PCR system is 100 μL, including 10 μL of 10×pfu Buffer, 4 μL of dNTPs (10 mmol / L), 4 μL of forward primer and reverse primer, plasmid as template: 20-30ng, and finally add pfu DNA polymerase 4μL, and finally add water to make up to 100μL. After configuring the components required for the PCR reaction, preheat the PCR instrum...
Embodiment 2
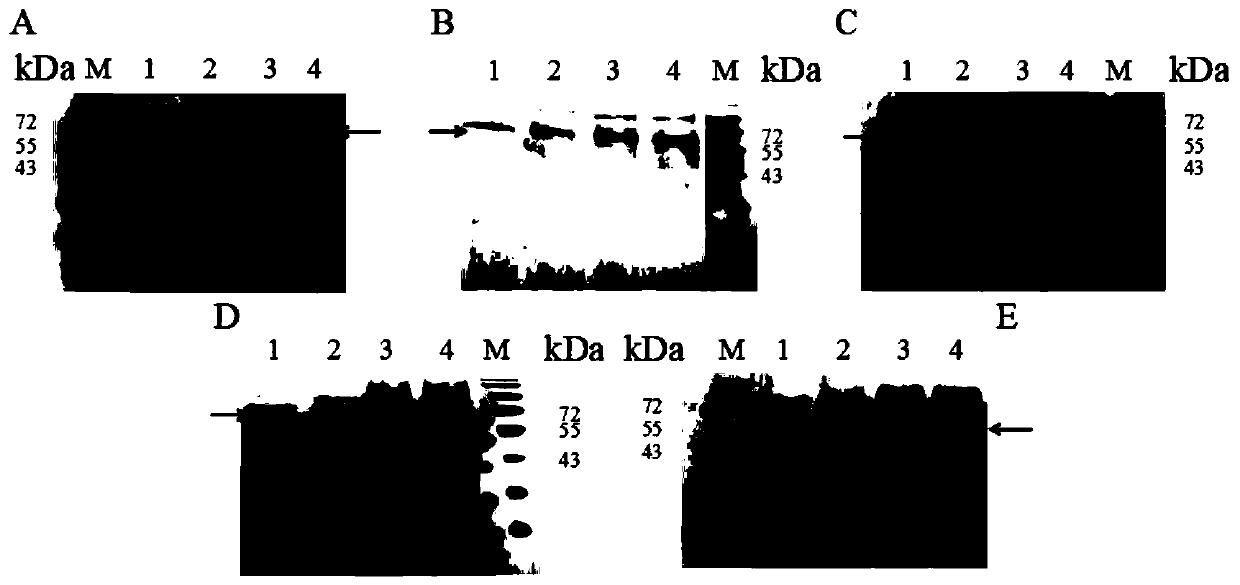
[0066] Example 2 Identification of cyclization ability of AEP cyclase
[0067] Step 1: Obtain the cyclization substrate of AEP cyclase
[0068] Since the SUMO protein is 11.0kDa in size, it can be well observed in SDS-PAGE and has the possibility of cyclization, so it is used as the cyclization substrate of AEP cyclase. Add GGGGSGGGGS to the N-terminal of the SUMO protease substrate, and add a longer Linker1 (GSGS), TEV protease substrate sequence (ENLYFQ / S), and a longer Linker2 (DVGGGGSEGGGSGGPGSGGEGSAGGGSAGGGS) to the C-terminal of the SUMO protease substrate in sequence and 6*His(HHHHHH), and finally add the recognition sequence of AEP cyclase at both ends to form a tandem substrate (GLP-SUMO protease substrate-Linker1-TEV protease substrate-Linker2-STRN / GLP-6*His ), the recognition sequence of AEP cyclase is shown in SEQ ID NO:5. Construct tandem substrates by overlap extension PCR, the forward primer is
[0069] SUMO-TEV-F: aactttaagaaggagatataccatgggcctgcaactgaaaggtt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com