Strain producing hydantoinase and carboxamide hydrolase and preparation method and application of strain
A hydantoinase and carbamyl technology, applied in the field of applied microorganisms, can solve the problem of low synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Determination of enzyme activity
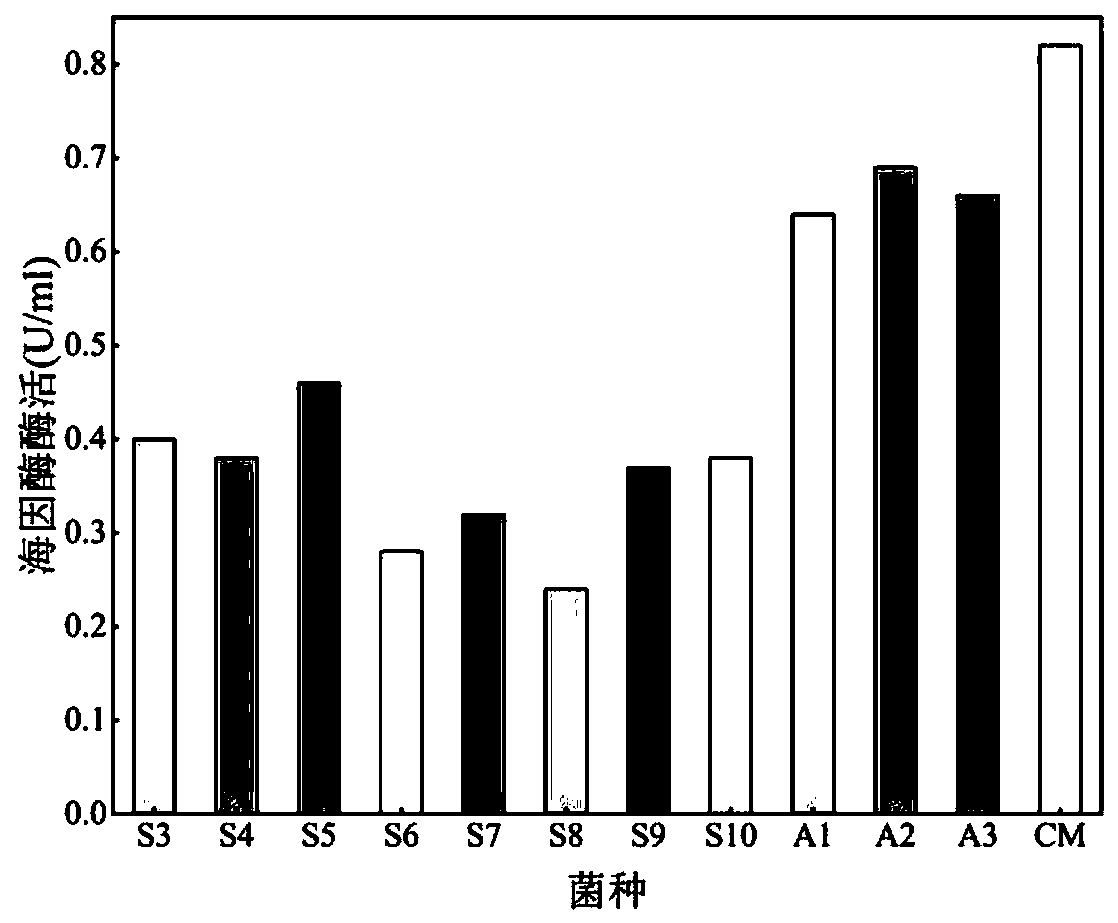
[0106] ①Definition of the activity of hydantoin enzyme with D,L-p-hydroxyphenylhydantoin as the substrate: the activity of 1 unit of hydantoin enzyme is defined as the reduction of 1 μmol D,L-p-hydroxybenzene per minute at pH 8.0, 45°C The amount of enzyme needed for hydantoin.
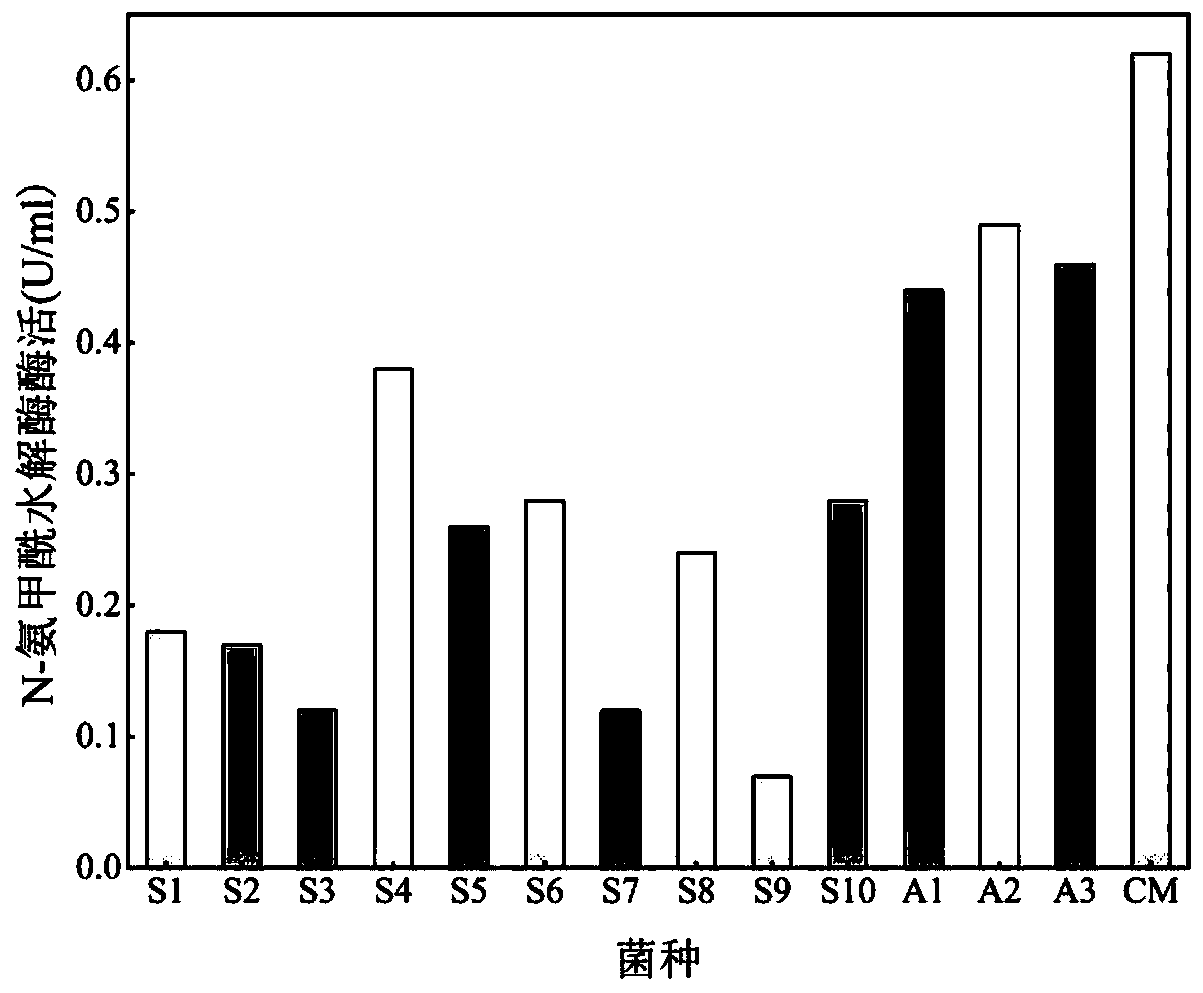
[0107] ②Definition of the activity of N-carbamyl hydrolase with N-carbamoyl-p-hydroxyphenylglycine as the substrate: the activity of 1 unit hydantoinase is defined as the reduction of 1 μmol N per minute at pH 8.0, 45°C - The amount of enzyme required for carbamoyl-p-hydroxyphenylglycine.
[0108] The determination method is as follows:
[0109] By analyzing the content of D-p-hydroxyphenylglycine and N-carbamoyl-p-hydroxyphenylglycine formed in the standard reaction, the activity of hydantoinase and N-carbamoylhydrolase in the soluble multi-enzyme extract was determined.
[0110] Standard reaction system for measuring hydantoin enzyme activity: D,L-p-hydro...
Embodiment 2
[0113] Study on Enzymatic Properties of Multi-enzyme Extract
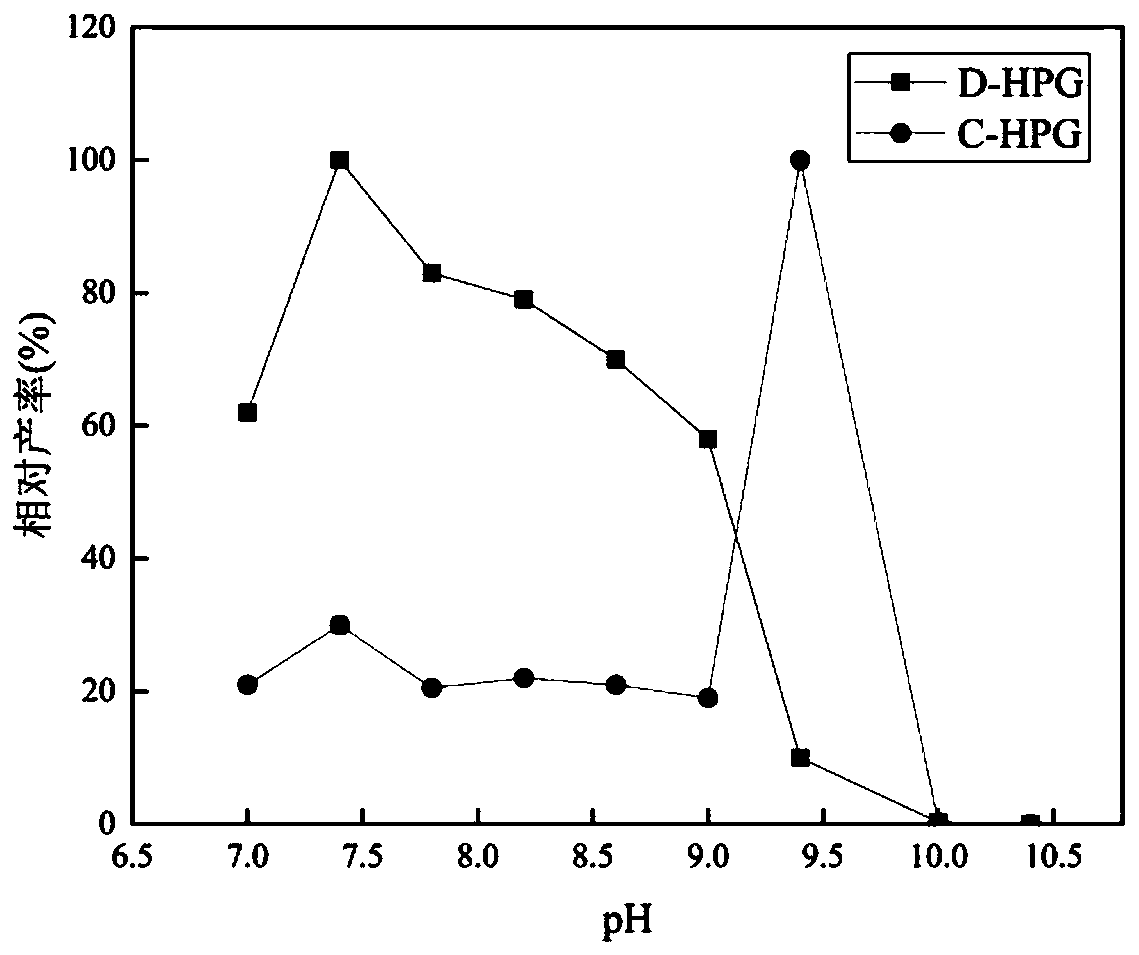
[0114] Optimum pH and pH Stability for Enzymatic Reactions
[0115] Take 0.1M buffer (pH range 7.0-10.4, pH 7.0-8.0 (0.1M Na 2 PO 4 ), pH8.0-9.0 (0.1M Tris-HCl), pH 9.0-10.4 (0.1M K 2 CO 3 )), reaction system: D, L-p-hydroxyphenylhydantoin (10g / L), dilute the enzyme solution, set up three parallels, and measure the generation of D-p-hydroxyphenylglycine and N-ammonia under the conditions of each pH value at 45°C Formyl p-hydroxyphenylglycine capacity. The highest production of D-p-hydroxyphenylglycine was taken as 100%, and the relative yield was plotted against pH.
[0116] Place the diluted enzyme solution in buffer solutions of different pH (pH range 7.0-10.4) at 45°C for 2 hours, set up three parallels for each, and measure the ability and N-carbamoyl-p-hydroxyphenylglycine, the highest D-p-hydroxyphenylglycine produced by the untreated enzyme was defined as 100%, and the relative yield was plotted agains...
Embodiment 3
[0121] In a 300L enzyme-catalyzed reactor, add 210kg of anaerobic water, then put in 32-38kg of D,L-p-hydroxyphenylhydantoin, stir at 65rpm, add wet enzyme preparation with an enzyme activity of 0.62U / ml for about 15kg, heat, control the temperature at 32-44°C, take samples at intervals of 3 hours and use high-pressure liquid chromatography to detect and reflect the situation. After the reaction is carried out for 6-8 hours, D-hydroxyphenylglycine crystals are precipitated, and D-hydroxyphenylglycine is specifically formed. The results of real-time monitoring conversion rate of phenylglycine are attached Figure 7 shown. When the N-carbamoyl-p-hydroxyphenylglycine in the reaction system is below 0.25%, the reaction is basically completed, and the conversion rate is above 99%. The product is obtained after partial refining of the crude crystal and the solution, and the total yield is 85%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com