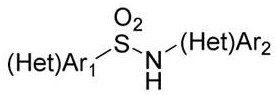
A kind of preparation method of sulfonamide compound
A technology for sulfonamides and compounds, applied in the field of preparation of sulfonamide compounds, can solve problems such as dependence, and achieve the effects of easy synthesis, good guiding significance and application prospects, and efficient synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
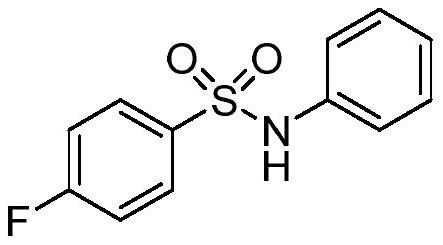
[0028] At room temperature, add 0.2 mmol of nitrosobenzene, 0.3 mmol of sulfur dioxide solid complex DABCO·(SO 2 ) 2 , 0.4mmol p-fluorophenyltetrafluoroborate diazonium salt, plug the reaction tube with a stopper and place it in high-purity argon to replace the gas, so that the system is in anhydrous and oxygen-free conditions, then add 2.0mL of dry acetonitrile and 0.4mmol of 1,4-Cyclohexadiene was placed in a heating device at 60°C and stirred for 12 hours. After the complete reaction was monitored by TLC, the reaction solution was concentrated under reduced pressure, and a mixture of 8:1 petroleum ether and ethyl acetate was used as the mobile phase for column chromatography separation to obtain the corresponding N-phenyl-4-fluorobenzene Sulfonamide 4-Fluoro-N-phenylbenzenesulfonamide Example 1.
[0029] Structural characterization of compound example 1: 1 H NMR (400MHz, Chloroform-d) δ7.81–7.73(m,2H), 7.30–7.21(m,2H), 7.17–7.03(m,5H), 6.72(s,1H). 13 C NMR (...
Embodiment 2
[0031]
[0032] At room temperature, add 0.2mmol of nitrosobenzene, 0.2mmol of sulfur dioxide solid complex DABCO·(SO 2 ) 2 , 0.3mmol p-methoxyphenyltetrafluoroborate diazonium salt, plug the reaction tube with a stopper and place it in high-purity argon to replace the gas, so that the system is in anhydrous and oxygen-free conditions, then add 4.0mL of dichloromethane and 0.2mmol of 1,4-cyclohexadiene was placed in a heating device at 40°C and stirred for 12h. After the complete reaction was monitored by TLC, the reaction solution was concentrated under reduced pressure, and a mixture of 4:1 petroleum ether and ethyl acetate was used as the mobile phase for column chromatography separation to obtain the corresponding N-phenyl-4-methoxy 4-Methoxy-N-phenylbenzenesulfonamide Example 2.
[0033] Structural characterization of compound example 2: 1 H NMR (400MHz, Chloroform-d) δ7.74(d, J=8.1Hz, 2H), 7.23(t, J=7.4Hz, 2H), 7.16(s, 1H), 7.09(d, J=7.6Hz ,3H),6.88(d,J=8.2Hz,2H),...
Embodiment 3
[0035]
[0036] At room temperature, add 0.2mmol p-chloronitrosobenzene, 0.4mmol sulfur dioxide solid complex DABCO·(SO 2 ) 2, 0.5mmol p-tolyl tetrafluoroborate diazonium salt, plug the reaction tube with a stopper and place it in high-purity argon to replace the gas, so that the system is in anhydrous and oxygen-free conditions, then add 2.0mL of dry acetonitrile and 0.6mmol of 1 , 4-cyclohexadiene, placed in a heating device at 30 ° C and stirred for 12 hours. After the complete reaction was monitored by TLC, the reaction solution was concentrated under reduced pressure, and a mixture of 6:1 petroleum ether and ethyl acetate was used as the mobile phase for column chromatography separation to obtain the corresponding N-(4-chlorophenyl) - p-toluenesulfonamide N-(4-chlorophenyl)-4-methylbenzenesulfonamide Example 3.
[0037] Structural characterization of compound example 3: 1 H NMR (400MHz, Chloroform-d) δ7.68(d, J=7.5Hz, 2H), 7.39(s, 1H), 7.24(d, J=7.7Hz, 2H), 7.18(d, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com