A method for preparing fluorescent silver nanoparticles based on silver mirror reaction
A technology of silver nanoparticle and silver mirror reaction, which is applied in nanotechnology, metal processing equipment, transportation and packaging, etc., to avoid the use of high temperature and high pressure and additional reducing agent, good fluorescence characteristics, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Take 0.06375g of silver nitrate in a clean beaker, add a small amount of distilled water to dissolve it completely, add concentrated ammonia water drop by drop until a brownish-yellow precipitate is formed, continue to add concentrated ammonia water until the precipitate is completely dissolved, and set the volume to 25mL to prepare 15 mmol L -1 of silver ammonia solution.
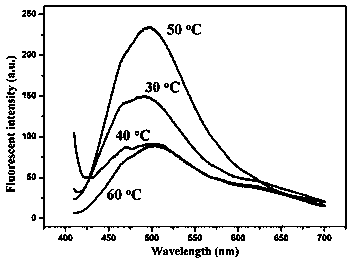
[0026] (2) Weigh 2.5 g of bovine serum albumin into a beaker, add water to dissolve it, and dilute it in a 50 mL volumetric flask to obtain a concentration of 50 mg mL -1 The bovine serum albumin solution, take 5 mL of the solution in four beakers, and then add 5 mL of the silver ammonia solution in step 1) respectively, and place them at 30, 40, 50, 60 o In a water bath of C, let it stand for 90 min. The reaction gave a light yellow solution, in which 30, 40 o The product under C is obviously turbid, 60 o The product at C was slightly cloudy.
[0027] (3) Transfer the above test solution t...
Embodiment 2
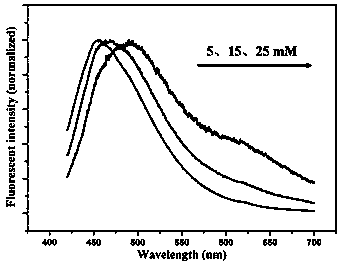
[0031] (1) Take 0.02125, 0.06375, and 0.10625 g of silver nitrate in a clean beaker, add a small amount of distilled water to dissolve it completely, add concentrated ammonia water drop by drop until a brownish-yellow precipitate is formed, continue to add concentrated ammonia water until the precipitate is completely dissolved, and set Volume up to 25mL, prepare 5, 15, 25 mmol L -1 of silver ammonia solution.
[0032] (2) Weigh 2.5 g of bovine serum albumin into a beaker, add water to dissolve it, and dilute it in a 50 mL volumetric flask to obtain a concentration of 50 mg mL -1 of bovine serum albumin solution, take 5 mL of this solution in a beaker, then add 5 mL of silver ammonia solution of different concentrations in step (1), and place it at 50 o In a water bath of C, let it stand for 90 min.
[0033] (3) Transfer the above test solution to a dialysis bag with a molecular weight cut off of 2000, and dialyze for 48 h. The dialyzed solution was taken on a molecular flu...
Embodiment 3
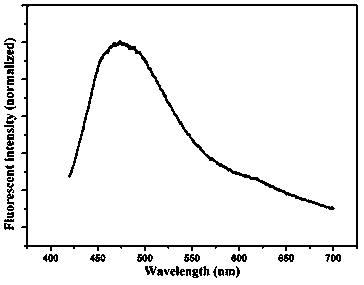
[0037] (1) Take 0.06375 g of silver nitrate in a clean beaker, add a small amount of distilled water to dissolve it completely, add concentrated ammonia water drop by drop until a brownish-yellow precipitate is formed, continue to add concentrated ammonia water until the precipitate is completely dissolved, and set the volume to 25 mL. Prepare 15 mmolL -1 of silver ammonia solution.
[0038] (2) Weigh 5.0 g bovine serum albumin into a beaker, add water to dissolve it, and then dilute it in a 50 mL volumetric flask to obtain a concentration of 100 mg mL -1 of bovine serum albumin solution, take 5 mL of this solution in a beaker, then add 5 mL of silver ammonia solution of different concentrations in step (1), and place it at 50 o In a water bath of C, let stand to react for 120 min.
[0039] (3) Transfer the above test solution to a dialysis bag with a molecular weight cut off of 2000, and dialyze for 48 h. The dialyzed solution was taken on a molecular fluorescence spectrom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com