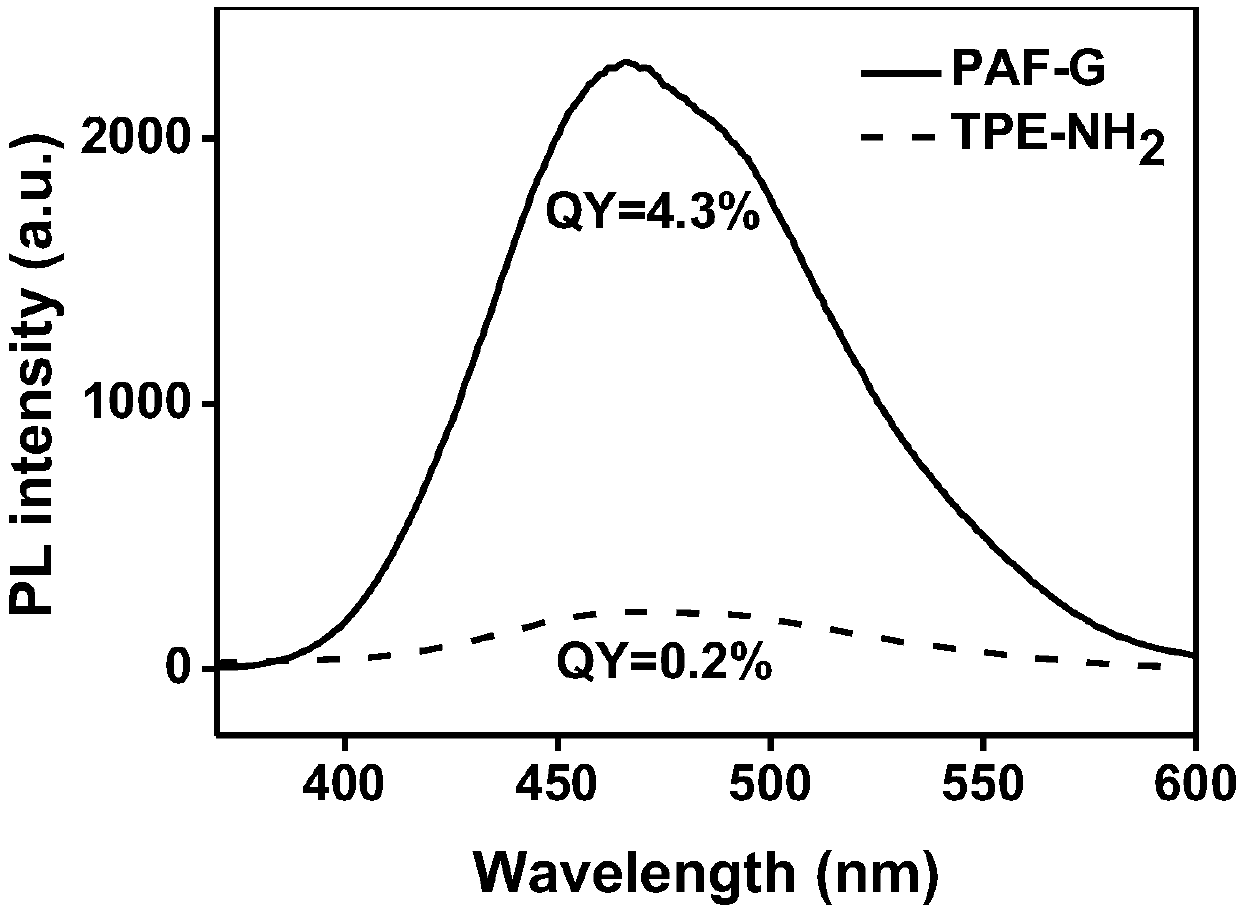
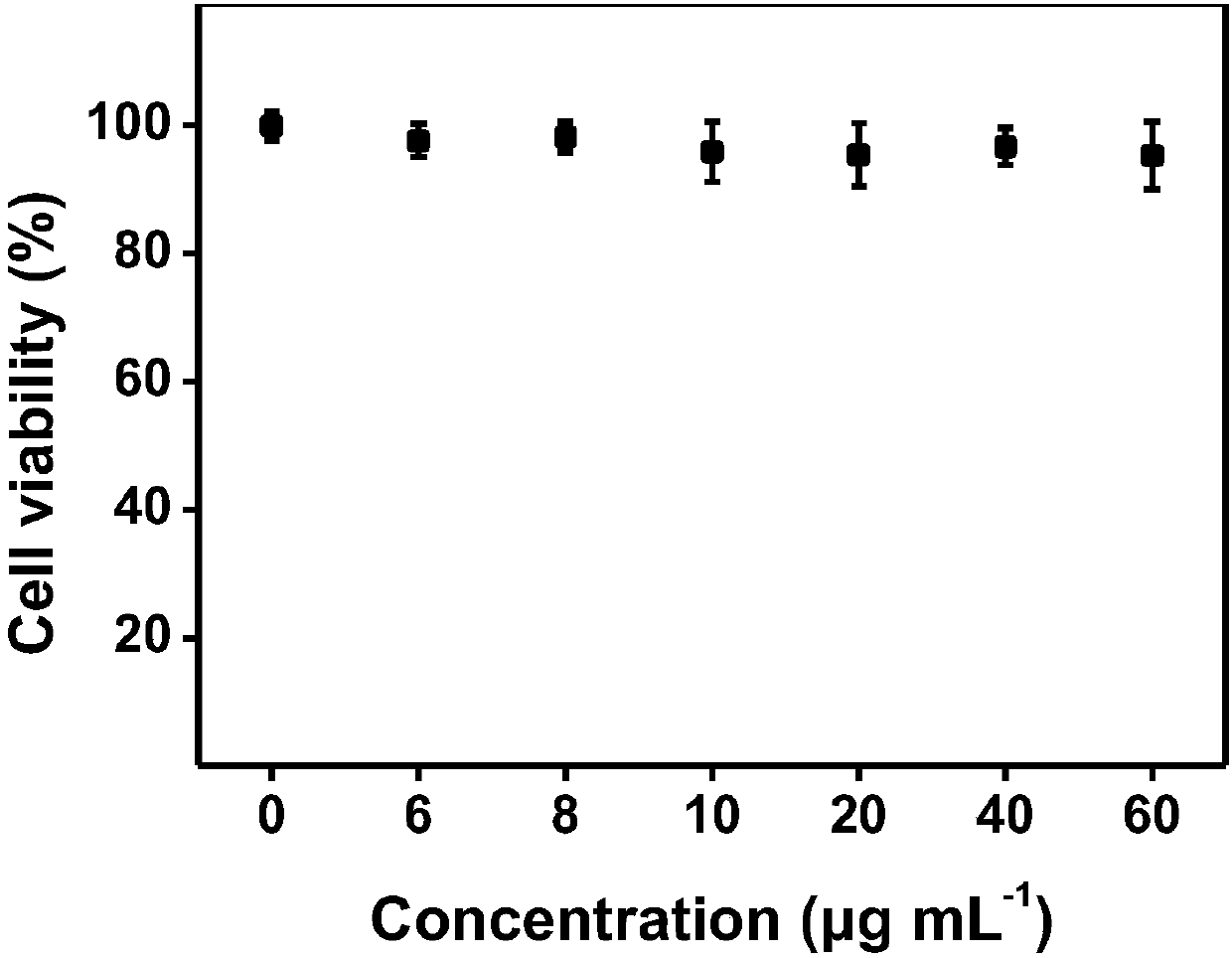
Amphiphilic polymer fluorescent material (PAF-G) and synthesis method thereof
A technology of amphiphilic polymers and fluorescent materials, applied in the field of water-soluble near-infrared fluorescent materials, amphiphilic polymer fluorescent materials and their synthesis, can solve the problems of limited use, insufficient stability of luminescent materials, etc., and achieve contrast high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] In this example, based on the methods of step 1 and step 2 of the present invention, alfa-amino polyethylene glycol with a chain length of 3 was used to synthesize and prepare the target molecule.
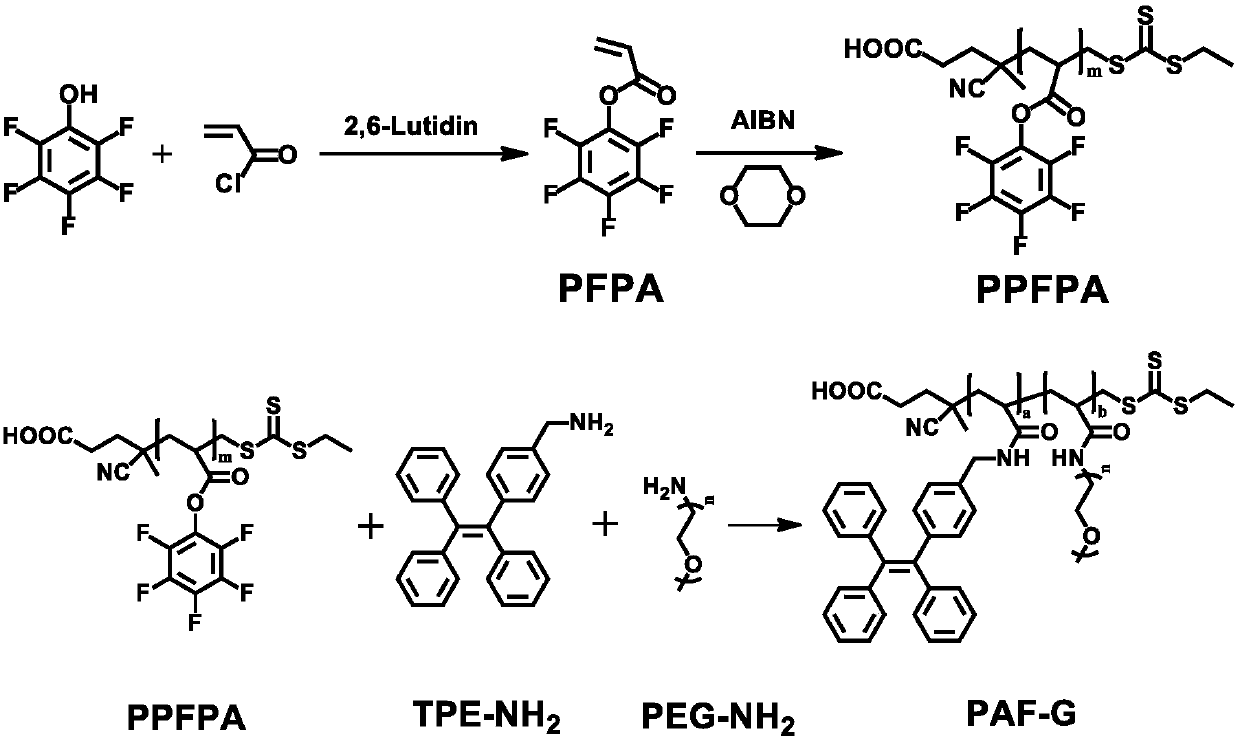
[0048] figure 1 The present invention relates to a synthetic route of PAF-G in an amphiphilic polymer fluorescent material and a synthetic method thereof.
[0049] In a round-bottomed flask, dissolve 2.5g of pentafluorophenol and 1.46g of 2,6-lutidine in dichloromethane, add 1.23g of acryloyl chloride to the mixture in an ice-water bath, and stir the reaction in an ice-water bath 3 hours, remove the ice-water bath and react at room temperature for 12 hours to obtain the crude product, filter with suction, save the filtrate, extract the filtrate with water twice, and use anhydrous MgSO 4 Carry out drying, remove solvent under reduced pressure, obtain crude product, use silica gel chromatography to crude product purification, obtain pentafluorophenol acryloyl chloride (PFPA),...
Embodiment 2
[0051] In this example, based on the methods of step 1 and step 2 of the present invention, alfa-amino polyethylene glycol with a chain length of 5 was used to synthesize and prepare the target molecule.
[0052] In a round-bottomed flask, dissolve 2.5g of pentafluorophenol and 2.91g of 2,6-lutidine in dichloromethane, add 3.69g of acryloyl chloride to the mixture in an ice-water bath, and stir the reaction in an ice-water bath After 5 hours, remove the ice-water bath and react at room temperature for 24 hours to obtain the crude product, filter it with suction, save the filtrate, extract the filtrate twice with water, and use anhydrous MgSO 4 Carry out drying, remove solvent under reduced pressure, obtain crude product, use silica gel chromatography to crude product purification, obtain PFPA, get 2.38g PFPA solution and 16.42mg azobisisobutyronitrile and 5.27mg4-cyano-4-(sulfur Benzoyl) valeric acid was mixed, the above reaction solution was freeze-thawed three times, and the...
Embodiment 3
[0055] In this example, based on the methods of step 1 and step 2 of the present invention, alfa-amino polyethylene glycol with a chain length of 9 was used to synthesize and prepare the target molecule.
[0056] In a round-bottomed flask, dissolve 2.5g of pentafluorophenol and 4.37g of 2,6-lutidine in dichloromethane, add 6.15g of acryloyl chloride to the above mixture, stir and react in an ice-water bath for 10 hours, remove React in an ice-water bath at room temperature for 48 hours to obtain a crude product, filter it with suction, save the filtrate, extract the filtrate twice with water, and use anhydrous MgSO 4 Carry out drying, remove solvent under reduced pressure, obtain crude product, use silica gel chromatography to crude product purification, obtain PFPA, get 2.38g PFPA solution and 32.84mg azobisisobutyronitrile and 26.34mg4-cyano-4-(sulfur Benzoyl) valeric acid was mixed, the above reaction solution was freeze-thawed three times, and the reactant was placed in an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com