An anti-human PD-1 monoclonal antibody preparation, combined drug and use thereof
A monoclonal antibody, PD-1 technology, applied in the direction of antibody, drug combination, drug delivery, etc., can solve problems such as difficult to apply PD-1 antibody, differences in physical and chemical properties and advanced conformation, and different amino acid sequence composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
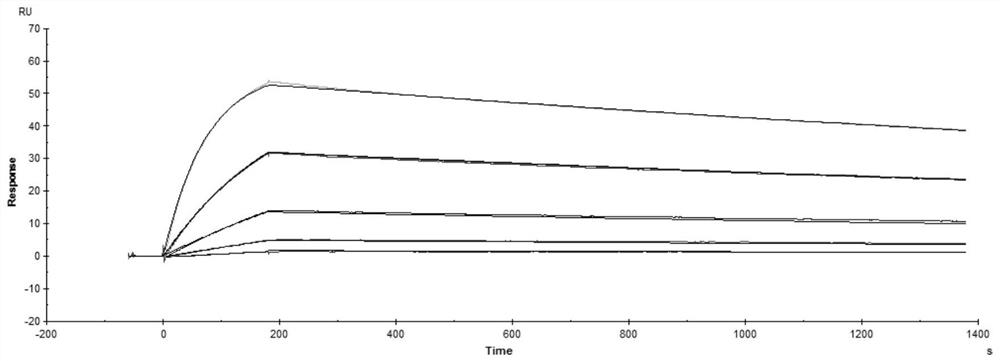
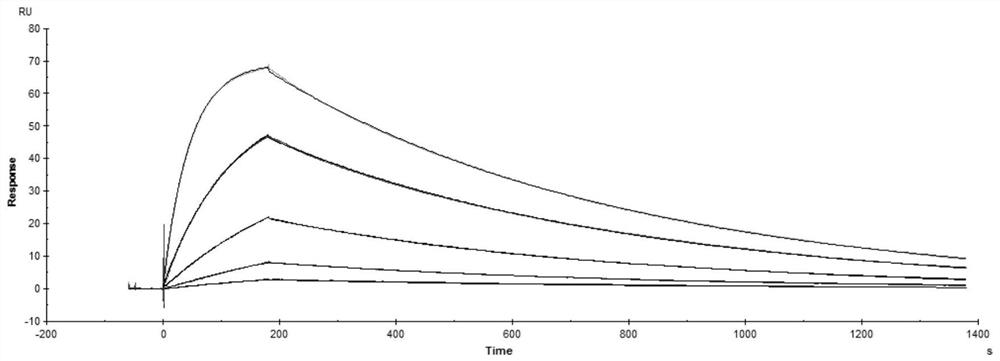
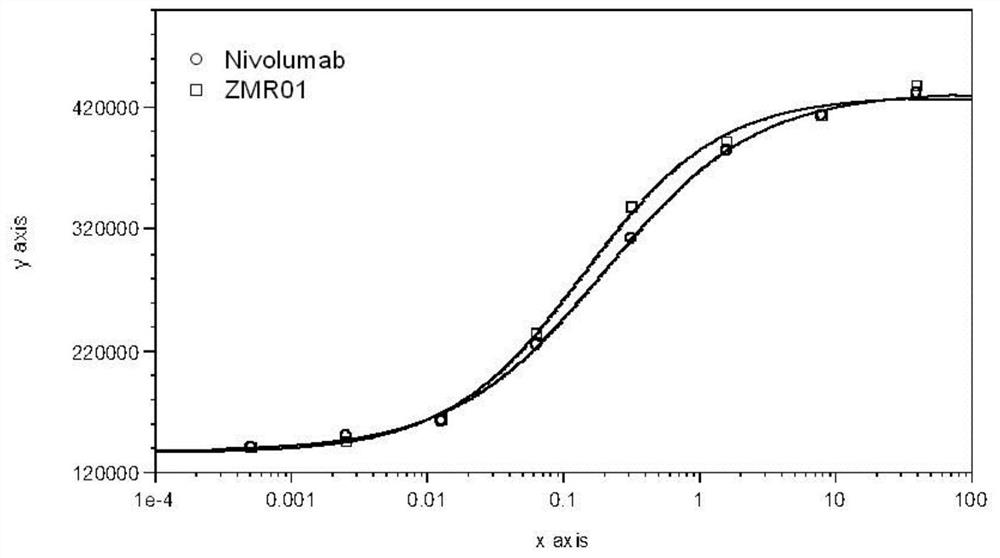
[0007] The purpose of the present invention is to provide a stable solution preparation suitable for the monoclonal antibody ZMR01 and its use.
[0008] The stable solution formulation of the present invention contains the monoclonal antibody ZMR01 or an antigen-binding fragment thereof and a buffer. The solution formulations may also contain stabilizers and / or surfactants.
[0009] In the solution preparation of the present invention, the HCDR sequences of the antibody heavy chain variable region of the monoclonal antibody ZMR01 or its antigen-binding fragment are as follows: SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO: 3; And, the antibody light chain variable region LCDR sequence is: SEQ ID NO:4, SEQ ID NO:5, SEQ ID NO:6. The amino acid sequence is shown in the following table:
[0010]
[0011] A further preferred monoclonal antibody ZMR01 has the heavy chain variable region of SEQ ID NO:7 and the amino acid sequence of the light chain variable region of SEQ ID NO:8:
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com