In situ drug delivery system constructed by pachymic acid A and preparation method and application of in situ drug delivery system
A technology of pachymic acid and drugs, which is applied in the field of in-situ drug delivery system constructed by pachymic acid A and its preparation, can solve the problems of less application development and research, and achieve the effects of strong cytotoxicity, diffusion prevention, and stable gel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation of PAA-DOX Gel drug-loaded gel
[0036] Use an analytical balance to weigh 10 mg of pachymic acid A into a 2 mL liquid phase vial, then add 1 mL of ethanol into the vial for 30 seconds of ultrasonication until completely dissolved, then add 700 μL of 4 mg / mL doxorubicin hydrochloride aqueous solution, and vortex Stable PAA-DOX Gel drug-loaded gel can be formed after mixing for 10 seconds and standing for 10 minutes.
Embodiment 2
[0037] Example 2 Preparation of PAA-DOX Gel drug-loaded gel
[0038] Use an analytical balance to weigh 10 mg of pachymic acid A into a 2 mL liquid phase vial, then add 1 mL of ethanol into the vial for 20 seconds of ultrasonication until it is completely dissolved, then add 700 μL of 4 mg / mL doxorubicin hydrochloride aqueous solution, and vortex Stable PAA-DOX Gel drug-loaded gel can be formed after mixing for 20 seconds and standing for 10 minutes.
Embodiment 3
[0039] Example 3 Preparation of PAA-DOX Gel drug-loaded gel
[0040] Use an analytical balance to weigh 10 mg of pachymic acid A into a 2 mL liquid phase vial, then add 1 mL of ethanol into the vial for 25 seconds of ultrasonication until completely dissolved, then add 700 μL of 4 mg / mL doxorubicin hydrochloride aqueous solution, and vortex Stable PAA-DOX Gel drug-loaded gel can be formed after mixing for 15 seconds and standing for 10 minutes.
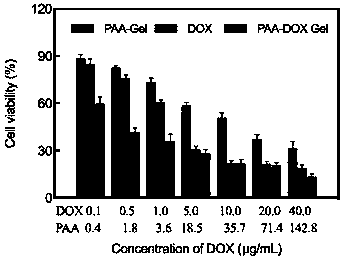
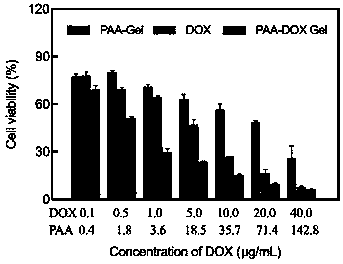
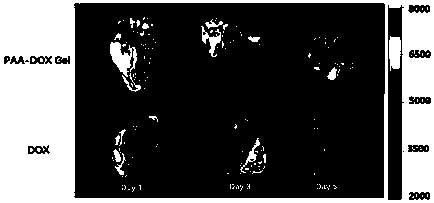
[0041] The following examples are the performance characterization of the above-mentioned drug delivery system and its application method and effect verification in drug delivery. The applied system is the PAA-DOX Gel drug-loaded gel prepared in Example 1:
[0042] Example 2 Characterization of PAA-DOX Gel drug-loaded gel
[0043] 1) Infrared spectroscopy to determine drug DOX loading
[0044] Infrared spectra of PAA-DOX Gel and PAA-Gel, DOX such as figure 1 As shown, in the process of drug gel formation, DOX interacts with PAA-Gel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com