Preparation method of impurities in synthesis of cabozantinib malate
A technology for cabozantinib malate and impurities is applied in the field of preparation of impurities in the synthesis of cabozantinib malate to achieve the effect of high impurity purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
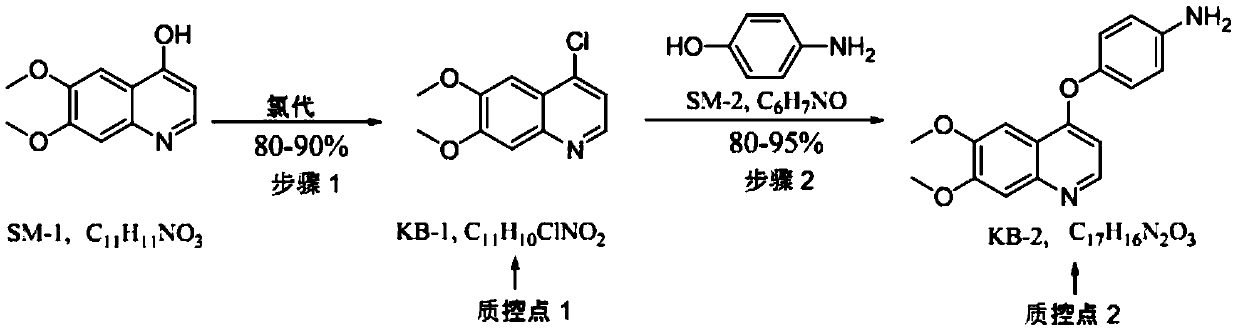
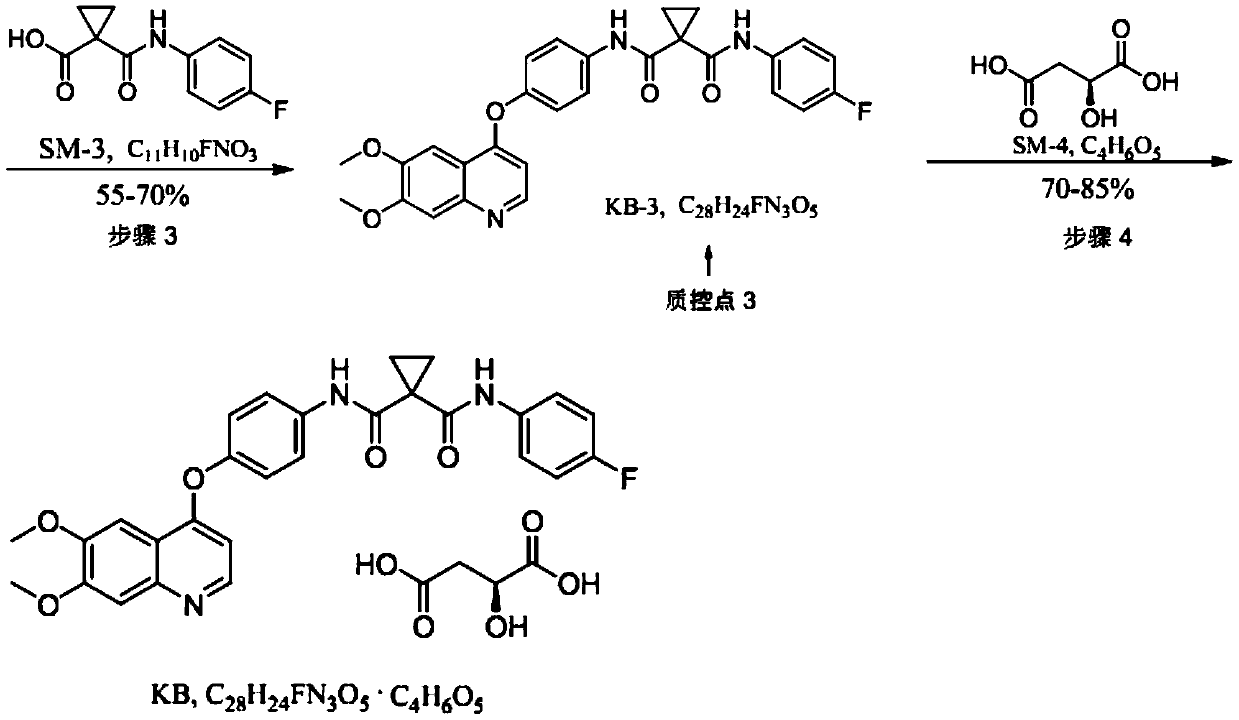
[0073] The impurity evolution process of cabozantinib malate:
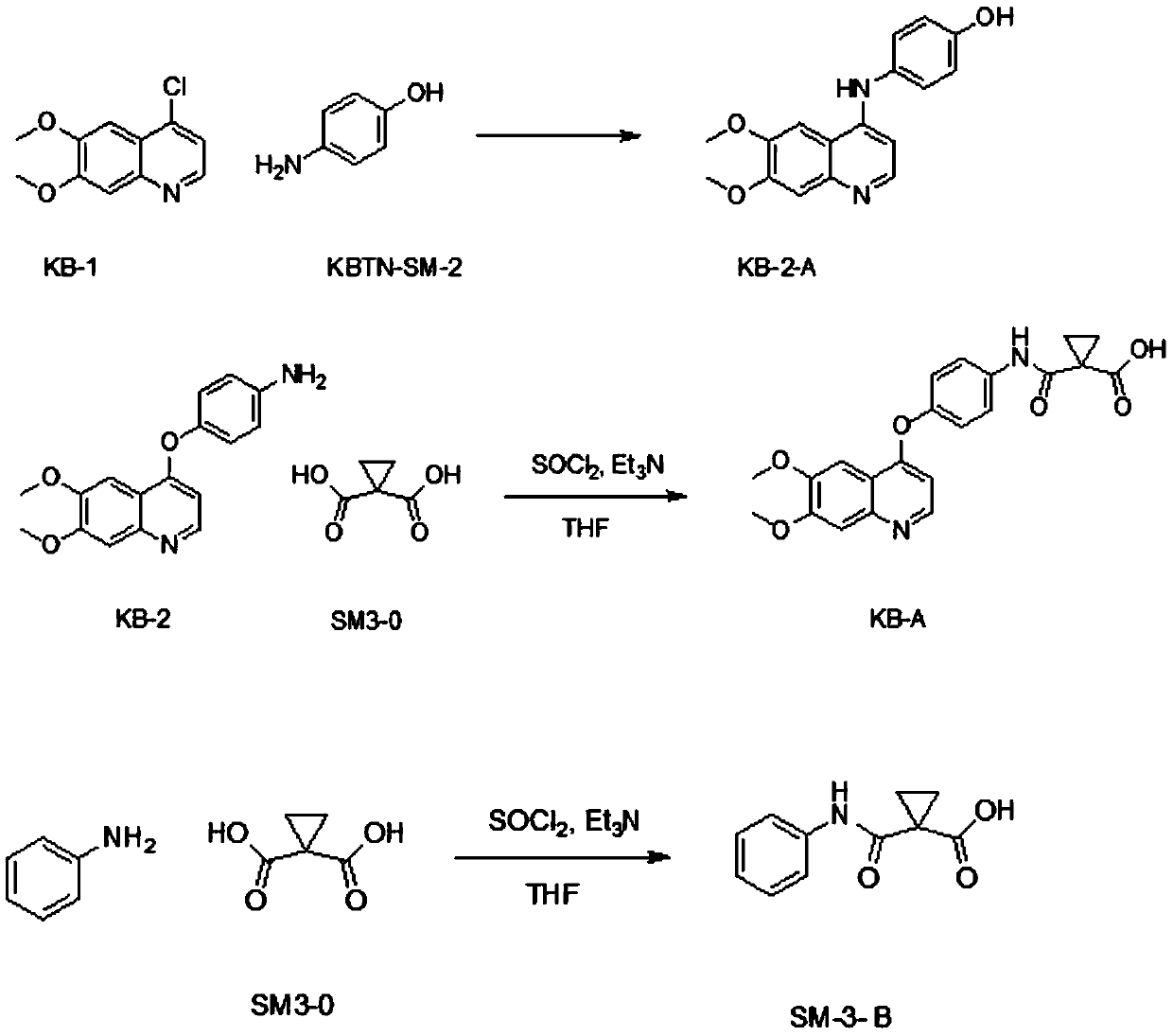
[0074] KB-2-A impurities: impurities introduced in step (2) nucleophilic substitution reaction.
[0075] In this step reaction, KB-1 and p-fluoroaniline release a nucleophilic substitution reaction under alkaline conditions. When they are different bases or different solvents, two products KB-2 and KB-2-A will be generated. , see the following reaction:
[0076]
[0077] The control method of this impurity is as follows: can control the generation of KB-2-A by controlling reaction conditions, under this reaction conditions (potassium tert-butoxide / dimethyl subalkali), the generation amount of this byproduct is very little, after passing through Disposal can be removed.
[0078] During the establishment of the analytical method and quality control of intermediate KB-2, the existence of impurity KB-2-A was considered. The level of this impurity can be controlled by available analytical methods. Since the impu...
Embodiment 2
[0091] The preparation method of said KB-2-A comprises the steps of:
[0092] Step a, add KB-1, KBTN-SM-2, and isopropanol to reaction vessel A in sequence, heat to reflux for 5 hours, lower to room temperature, and stir for 14 hours;
[0093] Step b, suction filtration, 80ml of isopropanol rinses the filter cake;
[0094] Step c, add 180ml of diethyl ether to reaction vessel B, filter cake, stir at room temperature for 1 hour, filter with suction, and blow dry at 45°C for 2.5 hours to obtain a yellow solid which is KB-2-A impurity.
[0095] In this example, 13 g of yellow solid was obtained, the product purity was 97%, and the yield was 49.0%.
Embodiment 3
[0097] The preparation method of said KB-2-A comprises the steps of:
[0098] Step a, add KB-1, KBTN-SM-2, and isopropanol to reaction vessel A in sequence, heat and reflux for 5-7 hours, lower to room temperature, and stir for 14-18 hours;
[0099] Step b, suction filtration, 80-120ml of isopropanol rinses the filter cake;
[0100] Step c, add 180-220ml ether to reaction vessel B, filter the cake, stir at room temperature for 1-3 hours, filter with suction, and air-dry at 45-55°C for 1.5-2.5 hours to obtain a yellow solid which is KB-2-A Impurities (KB-2-A4-[(6,7-Dimethoxy-4-quinolyl)amino]phenol CAS: 748707-58-6 Molecular weight: 296.32); KB-1, KBTN-SM-2 and The feeding ratio of isopropanol is 20g: 12g: 200ml.
[0101] In this example, 12 g of yellow solid was obtained, the product purity was 96%, and the yield was 45.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com