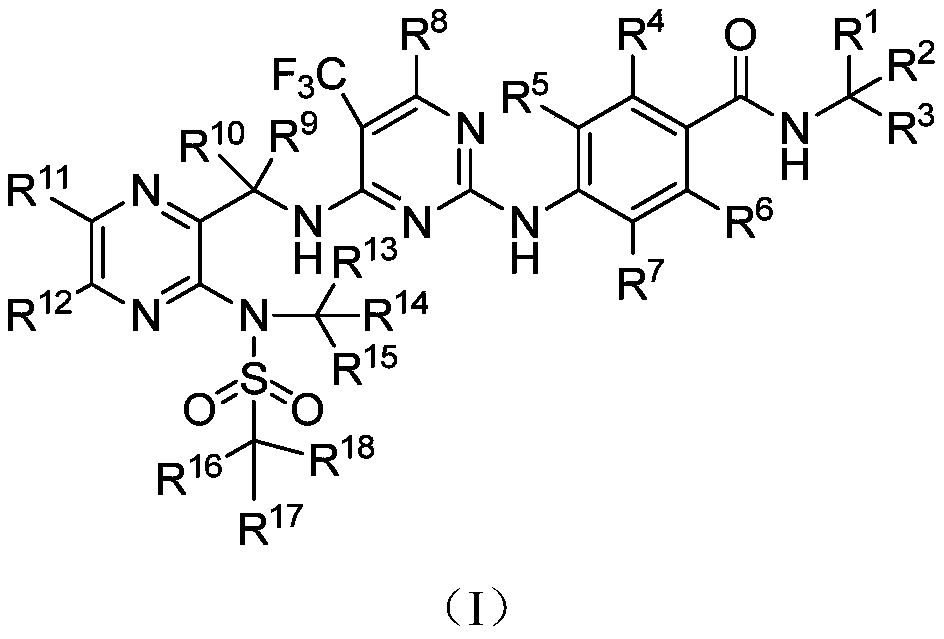
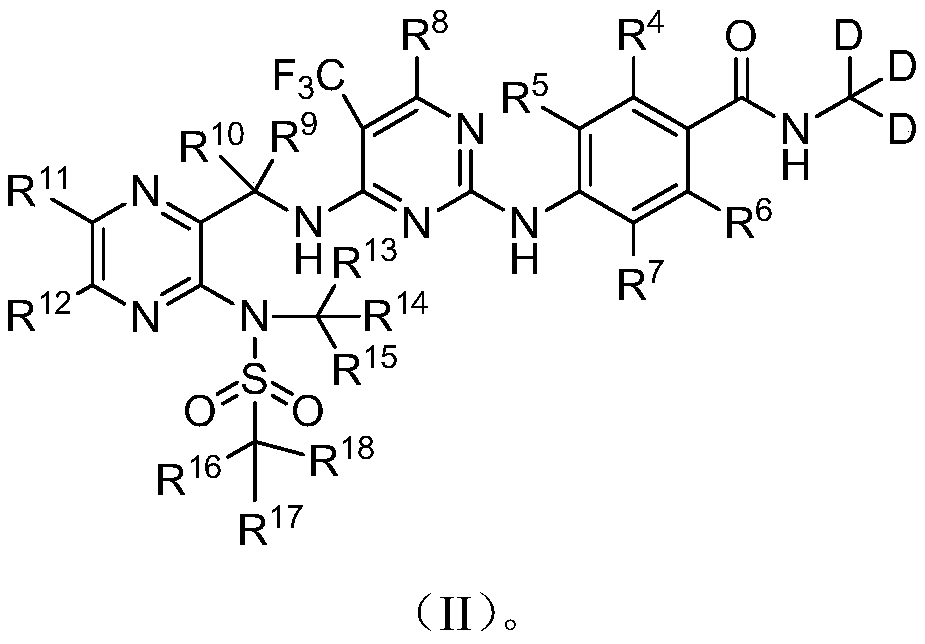
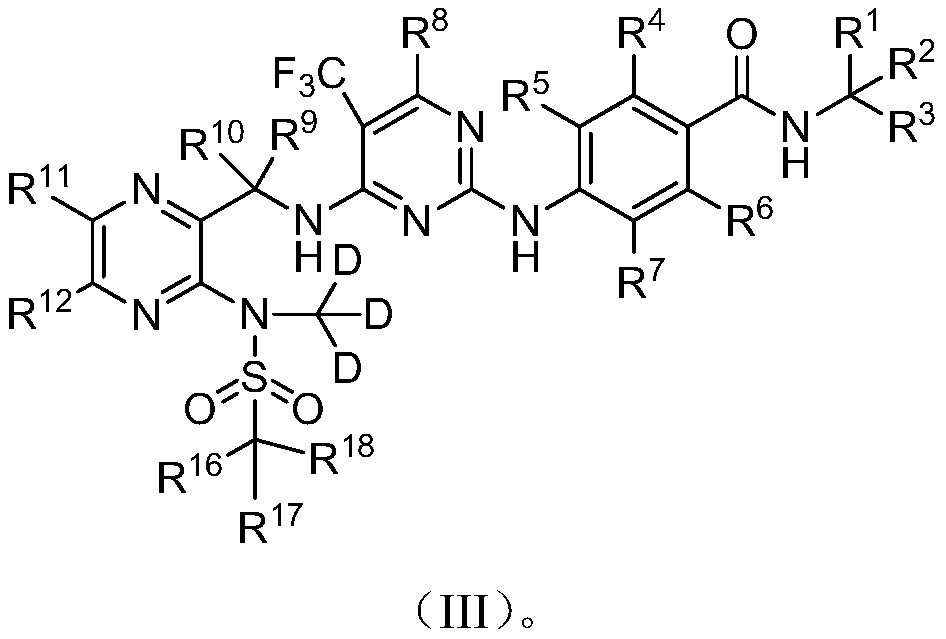
Deuterated Defactinib compounds and application thereof
A technology for compounds and uses, applied in organic chemistry, drug combinations, organic chemistry methods, etc., can solve problems such as unpredictable deuterium effects, and achieve the effects of improved metabolic stability and pharmacokinetic properties and excellent application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1, N-(trideuteromethyl)-4-((4-(((3-(N-methylmethylsulfonyl)pyrazin-2-yl)methyl)amino)-5- Synthesis of (trifluoromethyl)pyrimidin-2-yl)amino)benzamide (Compound 1)
[0042]
[0043] (1) Compound tert-butyl (4-((tert-butoxycarbonyl)(4-((tert-butoxycarbonyl)((3-(N-methylmethylsulfonyl)pyrazin-2-yl)methyl )amino)-5-(trifluoromethyl)pyrimidin-2-yl)amino)benzoyl)(methyl)carbamate
[0044]
[0045] N-methyl-4-((4-(((3-(N-methylmethylsulfonyl)pyrazin-2-yl)methyl)amino)-5-(trifluoromethyl)pyrimidine-2 -yl)amino)benzamide (200.0mg, 0.39mmol; compound 1-1, purchased from Chengdu Henghui Chemical Technology Co., Ltd.), DMAP (1.3g, 10.57mmol) was added to 10mL of dichloromethane, and then drop (Boc) 2 O (1.7 g, 7.83 mmol). The system was refluxed in an oil bath at 45°C for 24 hours. The next day, cool to room temperature, add dichloromethane and HCl (0.1M) solution, extract, leave to stand and separate layers, the organic phase is washed with saturated brine, drie...
Embodiment 2
[0053] Example 2, N-methyl-4-((4-(((3-(N-deuteromethylmethanesulfonamido)pyrazin-2-yl)methyl)amino)-5-(trifluoro Synthesis of Methyl)pyrimidin-2-yl)amino)benzamide (Compound 2)
[0054]
[0055] (1) Synthesis of compound N-deuteromethylmethanesulfonamide
[0056]
[0057] Weighed methanesulfonyl chloride (3.0g, 26.19mmol) into a 100mL single-neck round bottom flask, and added dichloromethane (30mL) into it, and stirred at room temperature to dissolve and clarify. Subsequently, the system was transferred to an ice-water bath to continue cooling and stirring. After 15 minutes, triethylamine (6.1 g, 60.24 mmol) was slowly added to the system. After the addition was complete, the system continued to insulate and stir for 10 minutes. Then, deuterated methylamine hydrochloride (2.0 g, 28.81 mmol) was slowly added in batches to the system. After completion, the ice bath was removed, and the system was allowed to return to room temperature and react overnight with stirring. ...
Embodiment 3
[0078] Example 3, N-(trideuteromethyl)-4-((4-(((3-(N-deuteromethylmethanesulfonamido)pyrazin-2-yl)methyl)amino)- Synthesis of 5-(trifluoromethyl)pyrimidin-2-yl)amino)benzamide (Compound 3)
[0079]
[0080] (1) Synthesis of compound tert-butyl (4-(deuteromethylcarbamoyl) phenyl) carbamate
[0081]
[0082] Weigh 4-((tert-butoxycarbonyl)amino)benzoic acid (1.1 g, 4.64 mmol) into a 100 mL single-neck round bottom flask, add 20 mL of DMF to it, and stir at room temperature. Subsequently, EDCI (1.3 g, 6.96 mmol), TEA (1.2 g, 11.6 mmol), deuterated methylamine hydrochloride (327.2 mg, 4.64 mmol), and DMAP (28 mg, 0.23 mmol) were successively added to the system. After completion, the system was stirred and reacted overnight at room temperature. The next day, after monitoring the consumption of raw materials, ethyl acetate (30mL) and water (20mL) were added to the system, stirred vigorously, and after standing to separate layers, the aqueous phase was back-extracted with eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com