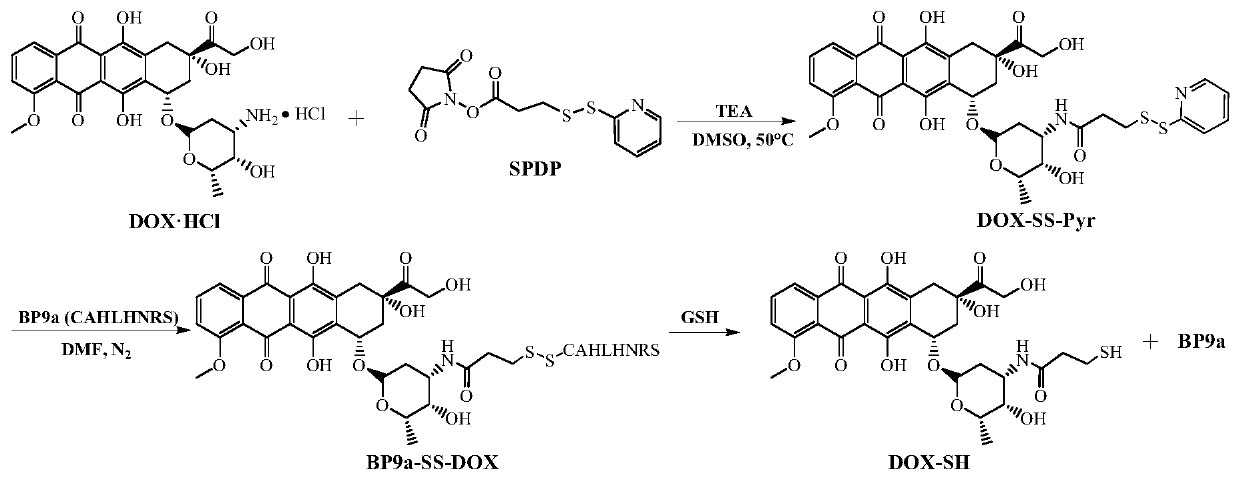
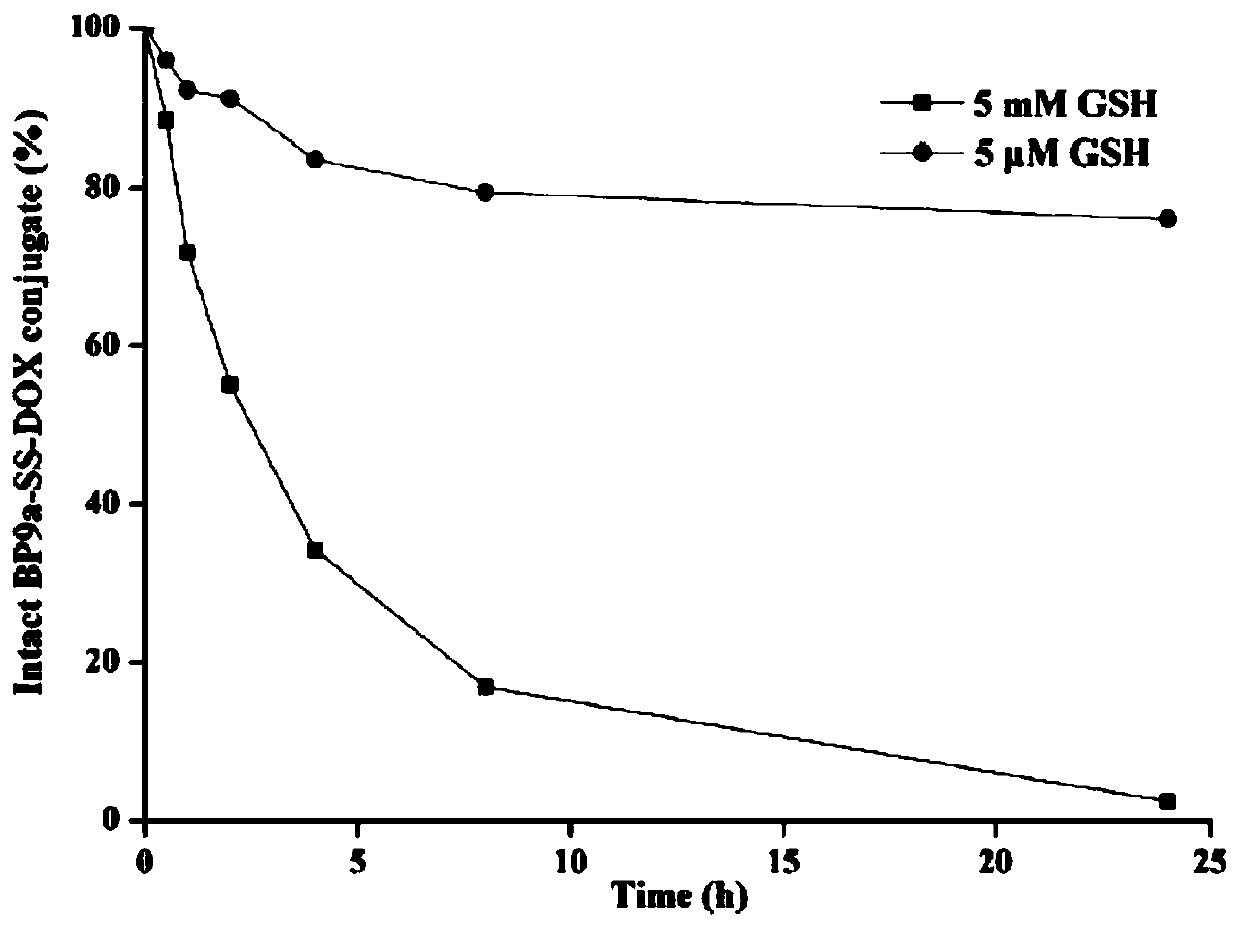
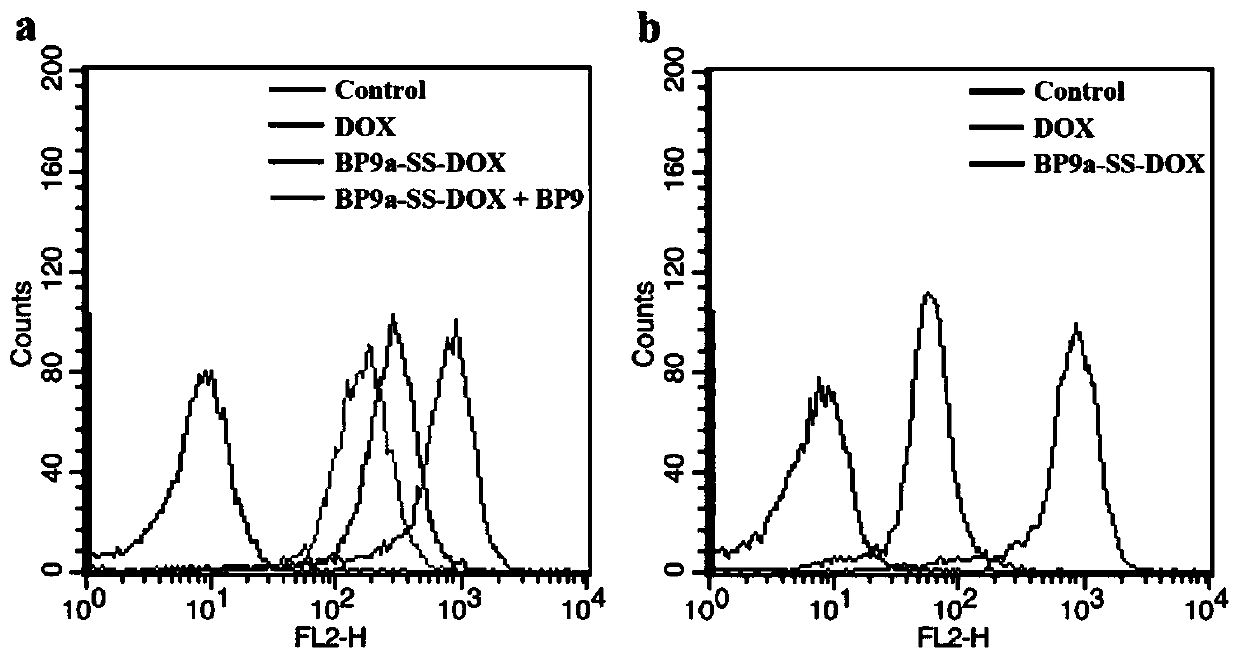
Transferrin receptor targeting polypeptide analogue-adriamycin conjugate and a preparing method and application thereof
A technology targeting polypeptides and transferrin, applied in the field of medicinal chemistry, can solve problems such as patient pain, toxic side effects, and clinical application limitations of doxorubicin, and achieve the goal of improving selectivity, reducing toxic side effects, and good tumor cell selectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Synthesis of TfR targeting peptide BP9a
[0058] Reference literature Li Songtao, Zhao Hongling, Yin Zhifeng, et al. Synthesis of transferrin receptor targeting peptide analog BP9a in liver cancer cells [J]. Chemical and Bioengineering. 2018, 35:27-29, 2-CTC resin ( 1.3mmol / g, 5mmol) reacted with Fmoc-Ser(tBu)-OH(15mmol) under the action of DIPEA(15mmol) to obtain Fmoc-Ser(tBu)-2-CTC resin, wherein 2-CTC resin and DIPEA and Fmoc- The molar ratio of Ser(tBu)-OH is 1:3:3; the resulting Fmoc-Pro-CTC resin is removed from the Fmoc protecting group with a volume concentration of 20% piperidine / DMF solution, and the ninhydrin test is positive, indicating that The Fmoc protecting group was successfully removed; using the Fmoc / tBu cross-protection strategy, using DIC(22.5mmol) / HOBt(22.5mmol) as the condensation reagent, according to the standard Merrifield polypeptide solid-phase synthesis method, sequentially condense the remaining Fmoc-amino acid (22.5mmol), wherei...
Embodiment 2
[0059] The reaction of embodiment 2 doxorubicin and SPDP
[0060] Referring to the records of Yoon S, Kim WJ, Yoo HS. Dual-responsive breakdown of nanostructures with high doxorubicin payload for apoptotic cancer therapy. Small. 2013, 9 (2): 284-293, weigh DOX·HCl (17.4mg, 30μmol) and SPDP (11.2mg, 36μmol) was dissolved in DMSO (5mL), then TEA (6.25μL, 45μmol) was added, the reaction was stirred at 50°C, and monitored by thin-layer chromatography (developing solvent: DCM:methanol, 13:1, v:v) Reaction process, after 6 hours of reaction, the target product DOX-S-S-Pyr was separated and purified by silica gel column chromatography (mobile phase: DCM:methanol, 20:1, v:v), and characterized by RP-HPLC and ESI-MS. RP-HPLC analysis purity is 95.7%; ESI-MS: m / z, [M+H] + : 741.5 (theoretical value), 741.3 (experimental value).
Embodiment 3
[0061] Example 3 Synthesis of BP9a-SS-DOX conjugates
[0062] BP9a (14.1 mg, 15 μmol) obtained in Example 1 and DOX-SS-Pyr (13.3 mg, 18 μmol) obtained in Example 2 were dissolved in anhydrous DMF (5 mL), wherein the molar ratio of BP9a to DOX-SS-Pyr 1:1.2;N 2 The reaction was stirred at room temperature under protection, and the reaction process was monitored by RP-HPLC. After the reaction was completed, the reaction solution was settled with glacial ether (50mL), and after centrifugation, the precipitate was washed with glacial ether (20mL×3), and finally the semi-preparative reversed-phase high-performance liquid chromatography was carried out. After purification and lyophilization, the target conjugate BP9a-SS-DOX was obtained. RP-HPLC analysis purity is 97.6%; ESI-MS: m / z, [M+H] + : 1566.7 (theoretical value), 1566.8 (experimental value); [M+2H] 2+ : 783.8 (theoretical value), 784.1 (experimental value).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com