Hepatitis E virus ORF2 protein
A technology of ORF2 and hepatitis E virus, which is applied in the field of vaccine protein screening, can solve problems such as interference with epidemic monitoring, difficulty in cultivating virus strains, and affecting the protection effect of virus attack, and achieves convenient and accurate detection methods, high antigen stability, and specificity strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1: Amplification and sequence analysis of ORF2 gene
[0015] In 2018, suspected hepatitis E occurred in some broiler breeders from Dalian area. After clinical investigation and laboratory testing, it was initially diagnosed as hepatitis E. After investigation, the above-mentioned infected chickens had been injected with hepatitis E vaccine and tested for neutralizing antibodies. It was suspected that the hepatitis E virus had mutated under the selection pressure of the vaccine, and the hepatitis E virus QD07 strain was isolated from the diseased chicken sample as a template for amplification.
[0016] 1. Amplification of the HEV ORF2 gene
[0017] According to the hepatitis E virus ORF2 gene sequence published in NCBI, primers were designed and synthesized. The sequence information of the primers is as follows:
[0018] primer1: 5′-ATGTCGGTGCGTGGATTGTTGCTC-3′;
[0019] primer2: 5'-CTAGGGTGGTGAGGGAAACGT-3'.
[0020] Extract the nucleic acid of the isolated...
Embodiment 2
[0027] Example 2: Recombinant expression of hepatitis E virus ORF2 protein
[0028] 1. Preparation method of ORF2 protein
[0029] The method comprises the following steps: a. constructing an expression vector; b. constructing an expression strain; c. inducing, extracting and purifying the recombinant ORF2 protein.
[0030] a. Construct expression vector:
[0031] The positive clone plasmid pMD18-T-ORF2 and the expression vector pET28a vector were digested with EcoRI and NotI, respectively, and the products were subjected to 1.2% agarose gel electrophoresis, and then recovered with a DNA gel recovery kit to obtain about 5400bp and 850bp fragments, respectively. The pET28a expression vector was constructed by directional ligation at 16°C; after the sequence and reading frame were verified by sequencing, the plasmid was linearized and transformed into BL21 competent cells.
[0032] b. Construction of expression strains, protein extraction and purification:
[0033] After tran...
Embodiment 3
[0035] Embodiment 3: the preparation of subunit vaccine
[0036] 1. Subunit vaccine preparation
[0037] 1. Preparation of ORF2 protein for seedling production
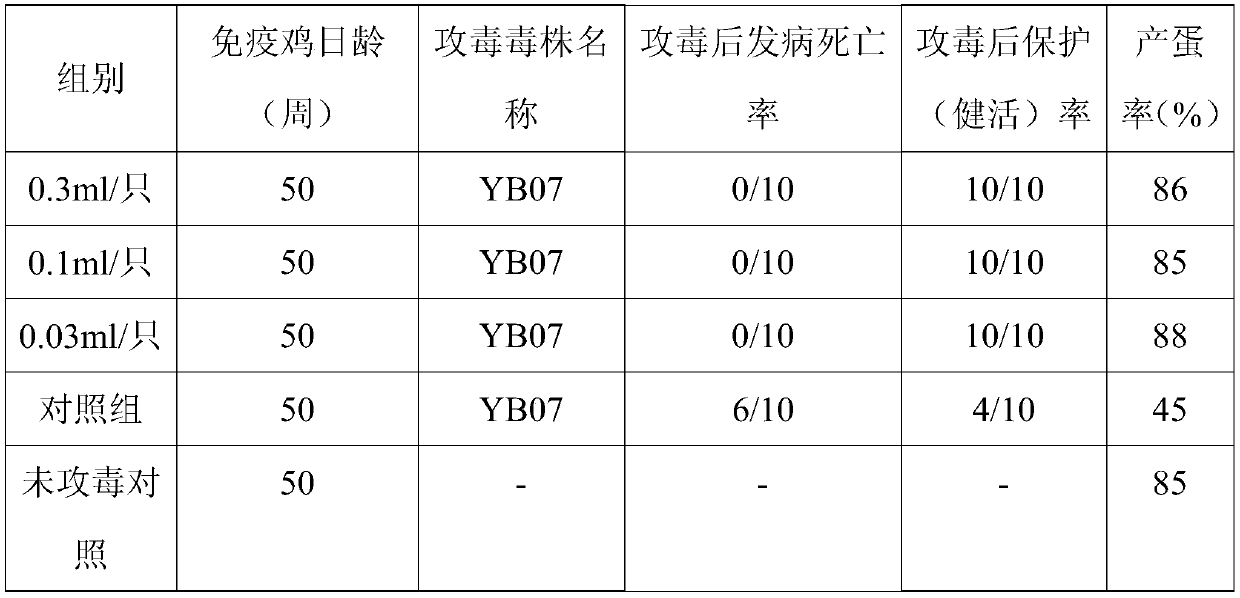
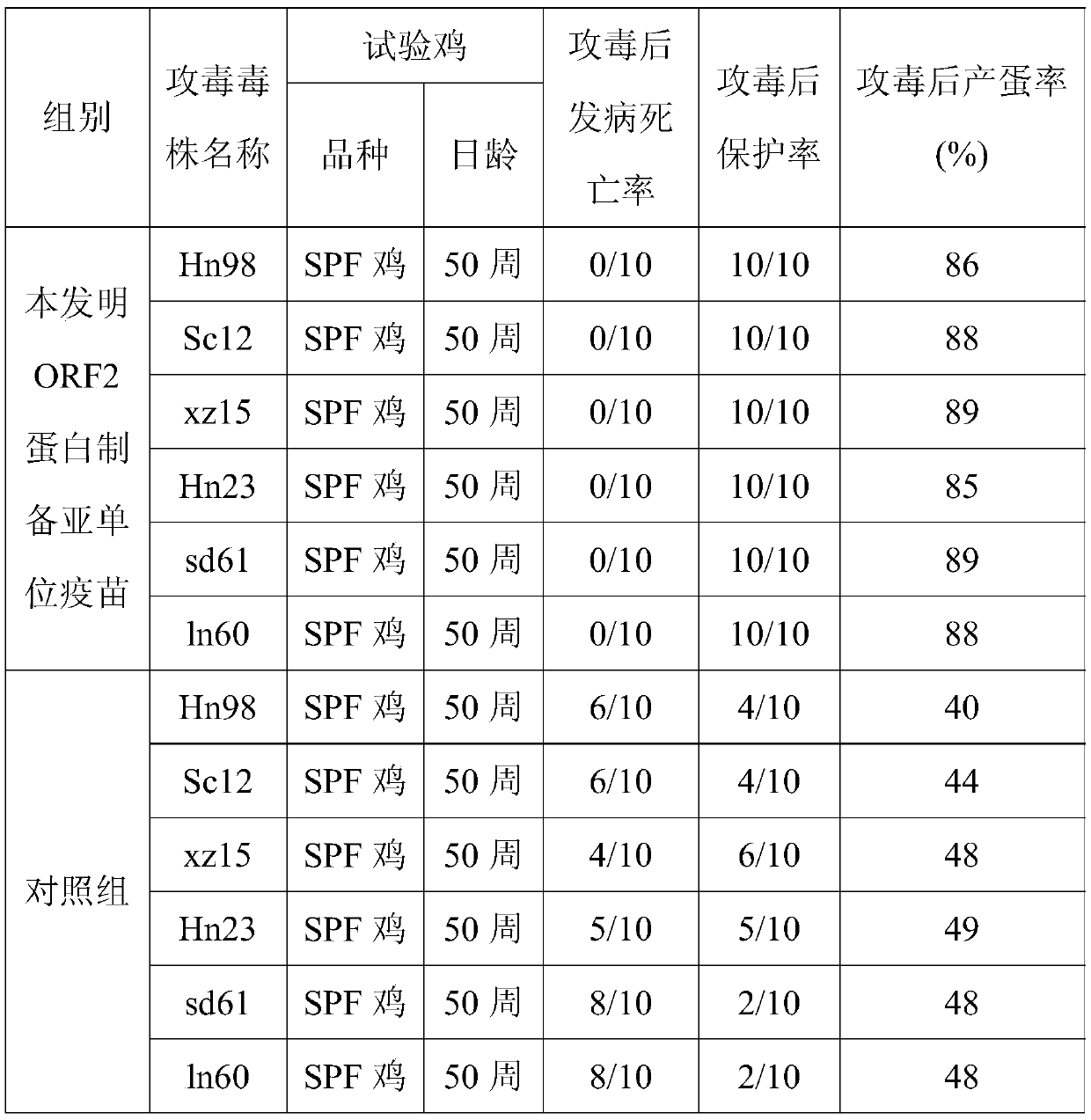
[0038] The BL21-ORF2 strain was inoculated in LB liquid medium containing kanamycin, cultured with shaking at 30°C for 18 hours, quantitatively distributed, and after pure inspection, it was used as the first-class seed. Take the first-grade seeds and inoculate them in LB liquid medium, culture them with shaking at 37°C for 18 hours, and store them at 2-8°C after microscopic examination. Add LB liquid medium according to 60% (V / V) of the volume of the fermenter, and at the same time add antifoaming agent according to 0.1% (V / V) of the medium, pass through high-temperature steam to sterilize for 30 minutes, and wait until the temperature of the medium drops to 37°C , inoculated with secondary seed solution for ORF2 protein production, the parameters of the fermenter were set to stir speed 800r / min, temperature 37°C, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com