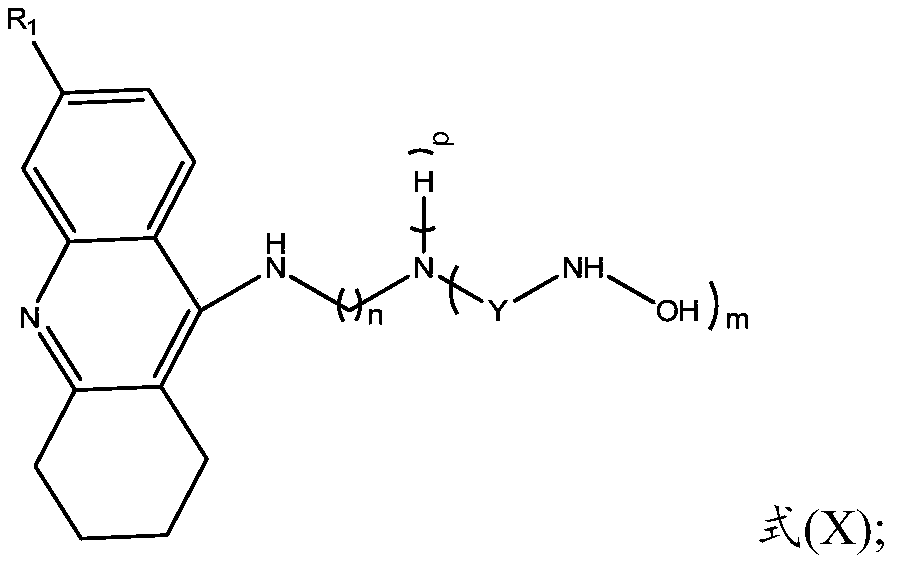
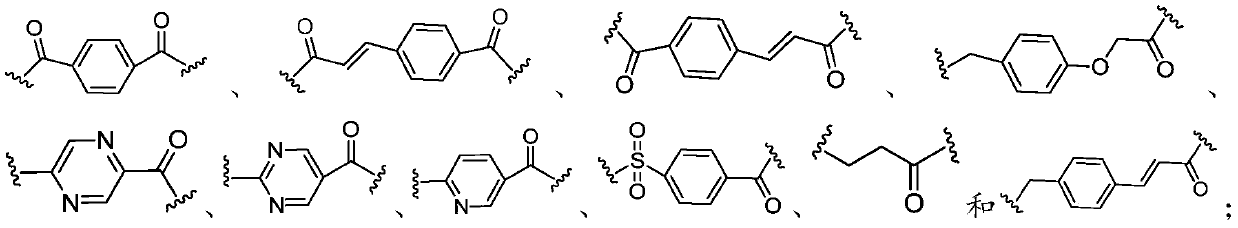
Multi-target tacrine derivative and preparation method and application thereof
A technology of pharmaceutical preparations and reactions, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Example 1: Synthesis of target compound H1-H7
[0136] Intermediate 2-aminobenzoic acid (6), 9-chloro-1, 2, 3, 4-tetrahydroacridine (8) and 6,9-dichloro-1, 2, 3, 4-tetrahydroacridine (9) Preparation
[0137] Add 2-aminobenzonitrile (5g, 42.3mmol) and potassium hydroxide (23.7g, 423mmol) into 30ml water, reflux at 100°C for 5h, cool to room temperature after the reaction, adjust pH=7-8 with dilute hydrochloric acid In between, intermediate 6 was obtained after drying to obtain 4.5g, intermediate 6 (2g, 16.9mmol) and cyclohexanone (2g, 20.3mmol) or intermediate 7 (2g, 11.65mmol) and cyclohexanone (2.28 g, 23.3mmol), slowly add 8ml of phosphorus oxychloride solution dropwise under ice bath conditions, and heat to reflux at 100°C for 6h. After the reaction is over, the reaction solution is slowly dropped into warm water after cooling to 60°C, stirring, intermediate 9. It can be precipitated directly, 3.5g after filtration and drying, and the intermediate 8 is adjusted to be ne...
Embodiment 2
[0193] Example 2: Synthesis of target compounds I1-I7
[0194] (E)-methyl 4-(3-oxo-3-((3-((1,2,3,4-tetrahydroacridin-9-yl)amino)propyl)amino)prop-1-en-1-yl) benzoate(19a)
[0195] (E)-4-(3-oxo-3-((3-((1,2,3,4-tetrahydroacridin-9-yl)amino)propyl)amino)prop-1-ene- 1-yl)methyl benzoic acid (19a)
[0196] First, (E)-3-(4-(methoxycarbonyl)phenyl)acrylic acid (0.16g, 0.784mmol) was dissolved in 10ml of dichloromethane, and TBTU (0.302g, 0.941mmol) was added to activate at room temperature for 40min, TLC Detection of activation is complete, Intermediate 12 (0.2g, 0.784mmol) is dissolved in dichloride and added to the activated acid, reacted at room temperature for 5h, TLC detects that the reaction is complete, the solid impurities in the reaction solution are filtered out, and the organic layer is collected. The organic layer was washed with saturated NaHCO3 aqueous solution (50ml*3), saturated brine (50ml*3), water (50ml*2), the organic layer was collected and dried over anhydrous magn...
Embodiment 3
[0236] Example 3: Synthesis of target compound J1-J4
[0237] methyl 3-((2-((1,2,3,4-tetrahydroacridin-9-yl)amino)ethyl)amino)propanoate(20a)
[0238] 3-((2-((1,2,3,4-tetrahydroacridin-9-yl)amino)ethyl)amino)propionic acid methyl ester (20a)
[0239] Dissolve Intermediate 10 (1g, 4.1mmol) in 12ml of tetrahydrofuran, add methyl 3-bromopropionate (0.7g, 4.1mmol), K2CO3 (1.7g, 12.3mmol), add 4ml of water, and heat at 70°C Reflux for 12h, TLC detects the completion of the reaction. When the reaction solution is cooled to room temperature, add 100ml of dichloromethane, wash with saturated brine (50ml*3), collect the organic layer, dry with anhydrous magnesium sulfate, evaporate under reduced pressure, and purify by column chromatography to obtain yellow The oily product 20a (0.8g) has a yield of 59.7%.
[0240] methyl 3-((2-((6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino)ethyl)amino)propanoate(20b)
[0241] Methyl 3-((2-((6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino)ethyl)amino)propionat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com