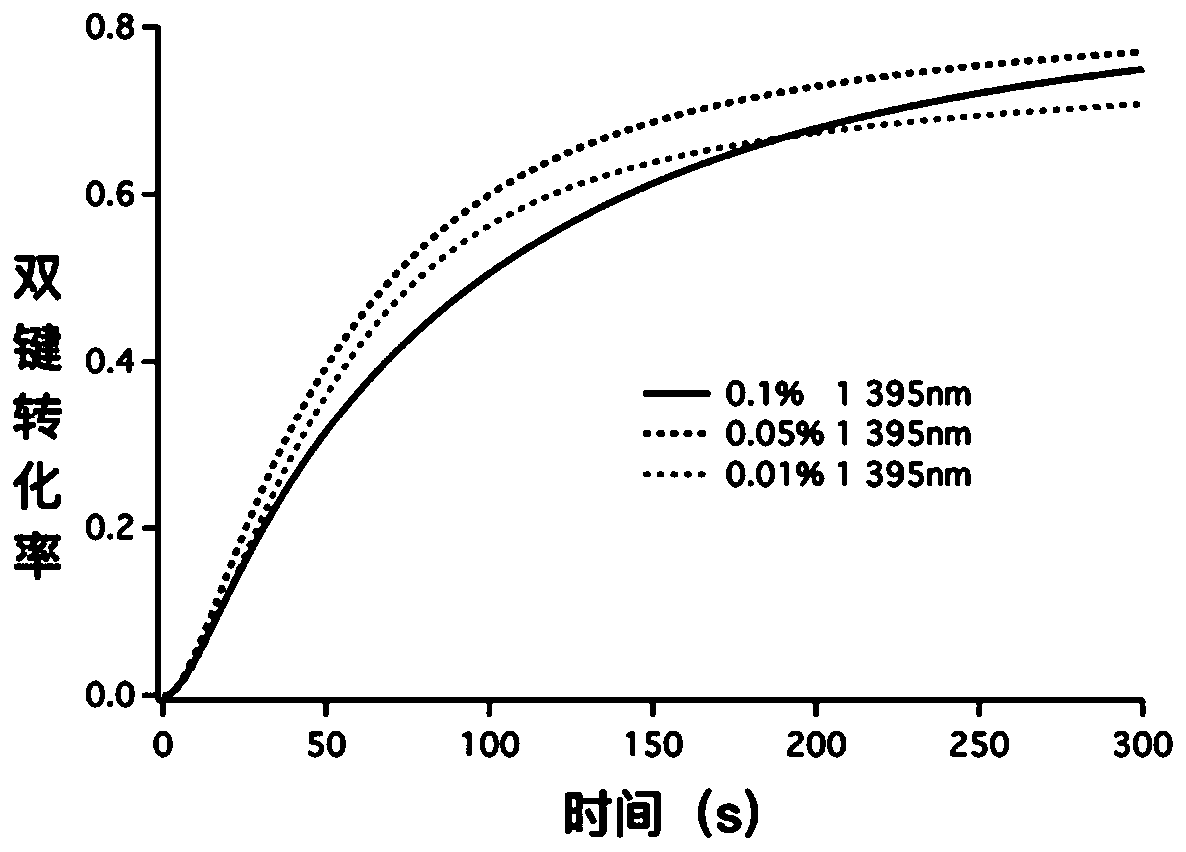
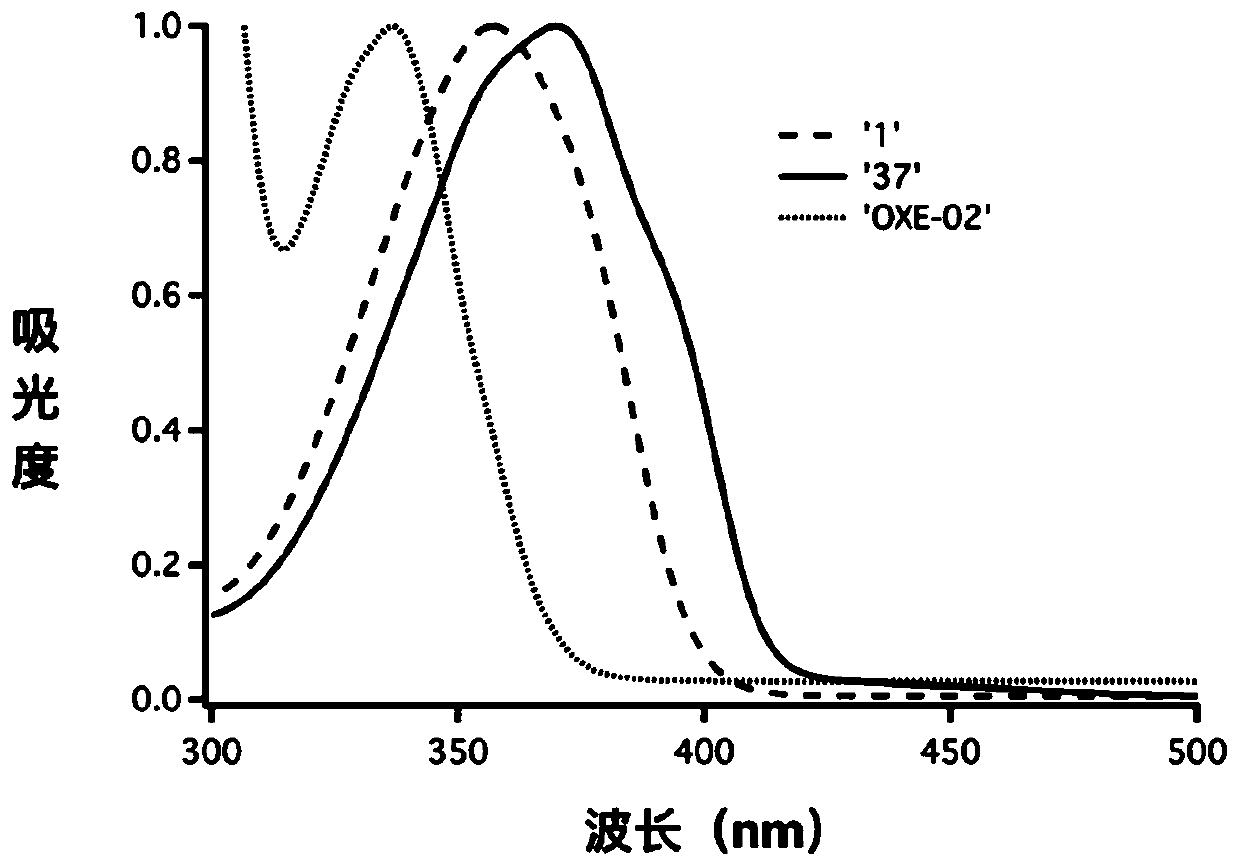
Oxime ester photoinitiators containing five-membered heteroaromatic ring structure, and preparation and use thereof
A technology of aromatic heterocycle and oxime ester, applied in the direction of organic chemistry, etc., can solve the problems of general stability, poor stability, difficult practical application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0136] Preparation Example 1: Preparation of Compound 1
[0137]
[0138] The synthetic route of compound 1 is as follows:
[0139]
[0140] Synthesis of intermediate compound 1a
[0141] 2,2'-dithiophene (0.08mol, 13.6g) was dissolved in 50ml of dichloroethane, then trifluoroacetic anhydride (0.7mol, 145g) and Mg(ClO 4 ) 2 (0.08mol, 17.87g), and the resulting mixture was heated to 80°C for 4h. Then stop the reaction, add 200g of ice, stir until the ice dissolves, add saturated aqueous sodium bicarbonate solution to neutralize the reaction solution until neutral. The organic phase was then extracted with dichloromethane and dried over anhydrous magnesium sulfate. After drying, it was filtered, and the organic phase was distilled off under reduced pressure to obtain a crude product, which was recrystallized from petroleum ether to obtain 17 g of the final product with a yield of 80%, which was identified as intermediate compound 1a. 1HNMR (400MHz, CDCl 3 , ppm) δ7.1...
preparation Embodiment 2-3
[0148] The method of Preparation Example 1 was repeated, and the reaction raw materials were appropriately changed to obtain compounds 2-3 and their NMR data in the following table respectively. Preparation Example 4: Preparation of Compound 4 Synthesis of Intermediate Compound 4a
preparation Embodiment 4
[0148] The method of Preparation Example 1 was repeated, and the reaction raw materials were appropriately changed to obtain compounds 2-3 and their NMR data in the following table respectively. Preparation Example 4: Preparation of Compound 4 Synthesis of Intermediate Compound 4a
[0149] 2,2'-dithiophene (0.027mol, 4.4g) was dissolved in 44ml of dichloroethane, then N,N-dimethylformamide (0.053mol, 3.9g) was added, and the POCl was added dropwise to the resulting mixture 3 (0.042mol, 6.3g), then stirred at room temperature for 5h. Then the reaction was stopped, and saturated aqueous sodium bicarbonate solution was added to neutralize the reaction solution to neutrality. The organic phase was then extracted with dichloromethane and dried over anhydrous magnesium sulfate. After drying, filter and remove the organic phase under reduced pressure to obtain a crude product, which is recrystallized with petroleum ether to obtain 3.9 g of the final product with a yield of 75%, wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com