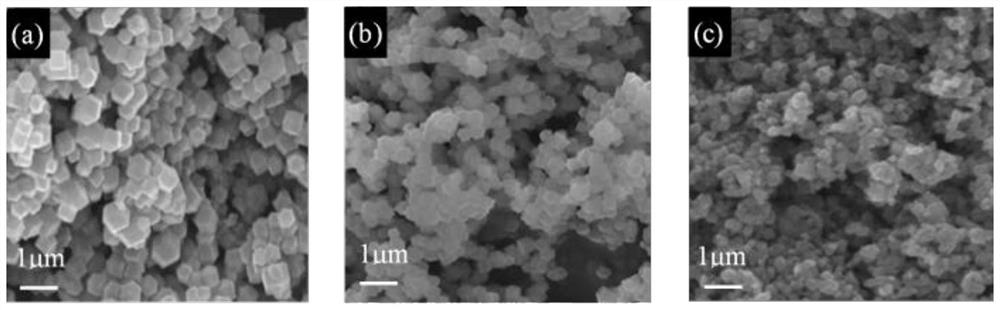
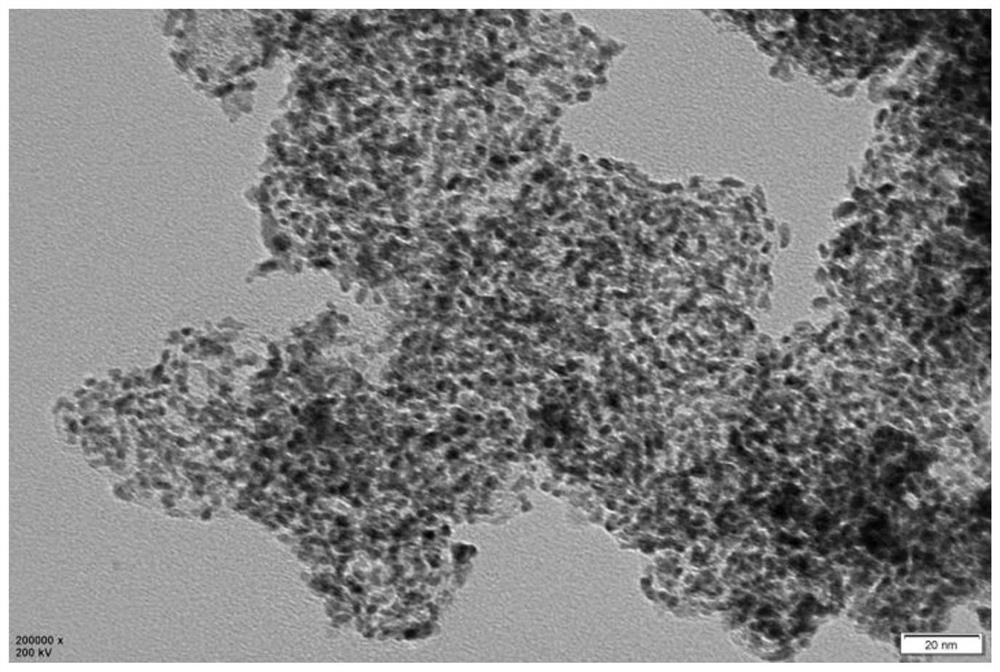
The preparation method of orr catalyst
A catalyst and metal-organic framework technology, applied in the field of ORR catalyst preparation, can solve problems such as unsatisfactory results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The technical scheme of the preparation method of the ORR catalyst of the present invention is as follows, comprising the following steps:
[0036] 1) Dissolve the cobalt salt and 2-(p-N-imidazolyl)phenyl-1H-4,5-imidazoledicarboxylic acid ligand in water and acetonitrile solvent, and add acid to adjust the pH value of the solution after ultrasonic and stirring 2 to 4, and then hydrothermal reaction occurs at a constant temperature of 140 to 155°C for 48 to 96 hours; after cooling to room temperature, the obtained product is washed in turn and dried naturally to form a black-green transparent cobalt-containing multi-nitrogen metal organic compound. Framework material precursor (chemical formula: [Co(p-IPhHIDC)]n);
[0037] 2) The precursor material [Co(p-IPhHIDC)] prepared in step 1) n , after being thoroughly ground, ultrasonically dispersed in chloroplatinic acid (H 2 PtCl 6 ·6H 2 O) in the ethylene glycol solution, this mixed solution is placed in the microwave sy...
Embodiment 1
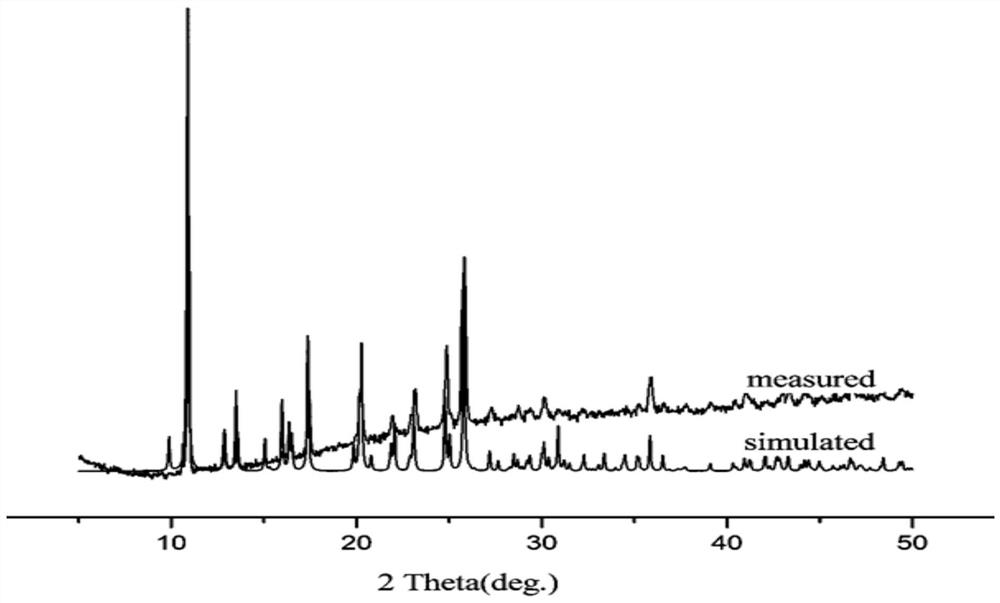
[0044] 1. 0.05mmol, 11.9mg of CoCl 2 ·6H 2 O and 0.03 mmol, 8.9 mg of 2-(p-N-imidazolyl)phenyl-1H-4,5-imidazoledicarboxylic acid ligand (p-IPhH3IDC) were dissolved in 2 mL of acetonitrile and 5 mL of water, mixed well and added concentrated HCl Adjust the pH to 2, and conduct a hydrothermal reaction at 150°C for 96 hours; after the reaction, the product obtained is centrifuged, washed three times with acetone, washed three times with water, and dried naturally at room temperature to obtain a black-green transparent cobalt-containing multi-nitrogen metal-organic framework Material. figure 1 Shown is the XRD pattern of the resulting product.
[0045] 2. After the material obtained in step 1 and the ethylene glycol solution of chloroplatinic acid are in a molar ratio of 1:0.02 ultrasonically dispersed, in N 2 Place it in a microwave synthesizer at 113°C under protection, and react with 10s / 10s intermittent microwave for 16min to make H 2 PtCl 6 Restored to simple Pt; in orde...
Embodiment 2
[0051] 1. 0.05mmol, 11.9mg of CoCl 2 ·6H 2 O and 0.03 mmol, 8.9 mg of 2-(p-N-imidazolyl) phenyl-1H-4,5-imidazole dicarboxylic acid ligand (p-IPhH3IDC,) were dissolved in 2 mL of acetonitrile and 5 mL of water, mixed well and added to HCl was adjusted to pH 4, and hydrothermal reaction was carried out at 150°C for 96 hours; after the reaction, the product obtained was centrifuged, washed three times with acetone, and washed three times with water, and dried naturally at room temperature to obtain a black-green transparent cobalt-containing multi-nitrogen metal organic compound. Framework material (Co-MNMOF).
[0052] 2. After the precursor Co-MNMOF material and the ethylene glycol solution of chloroplatinic acid are dispersed uniformly by ultrasonic at a molar ratio of 1:0.05, the N 2 Place it in a microwave synthesizer at 113°C under protection, and react with 10s / 10s intermittent microwave for 16min to make H 2 PtCl 6 Restored to simple Pt; in order to prevent the metal (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com