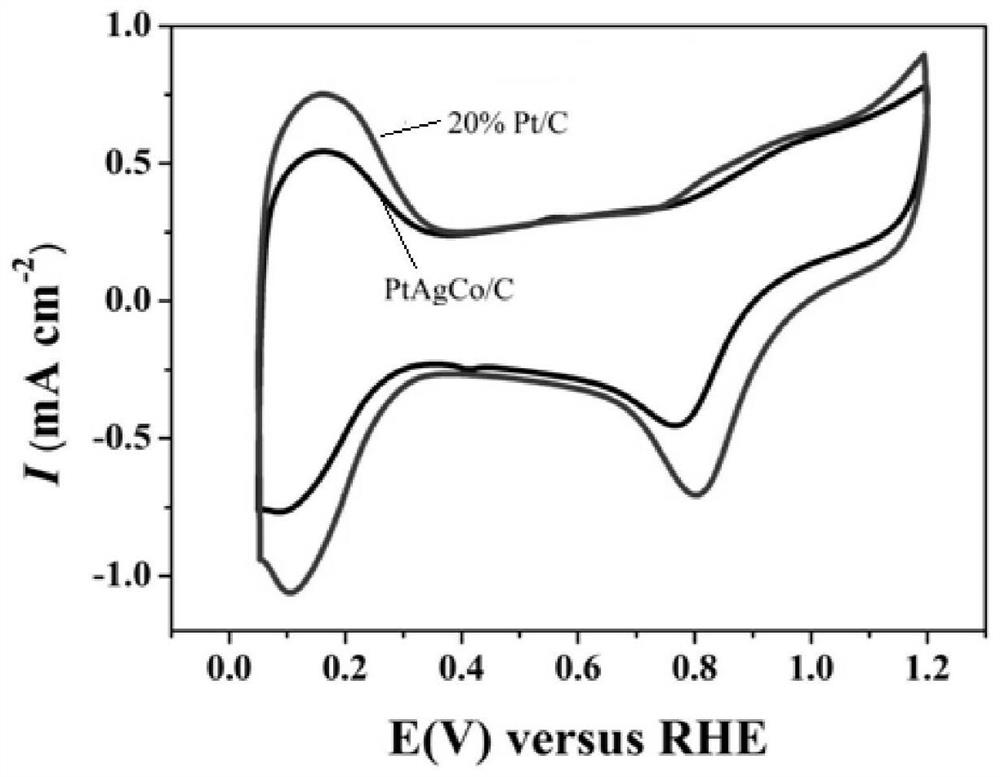
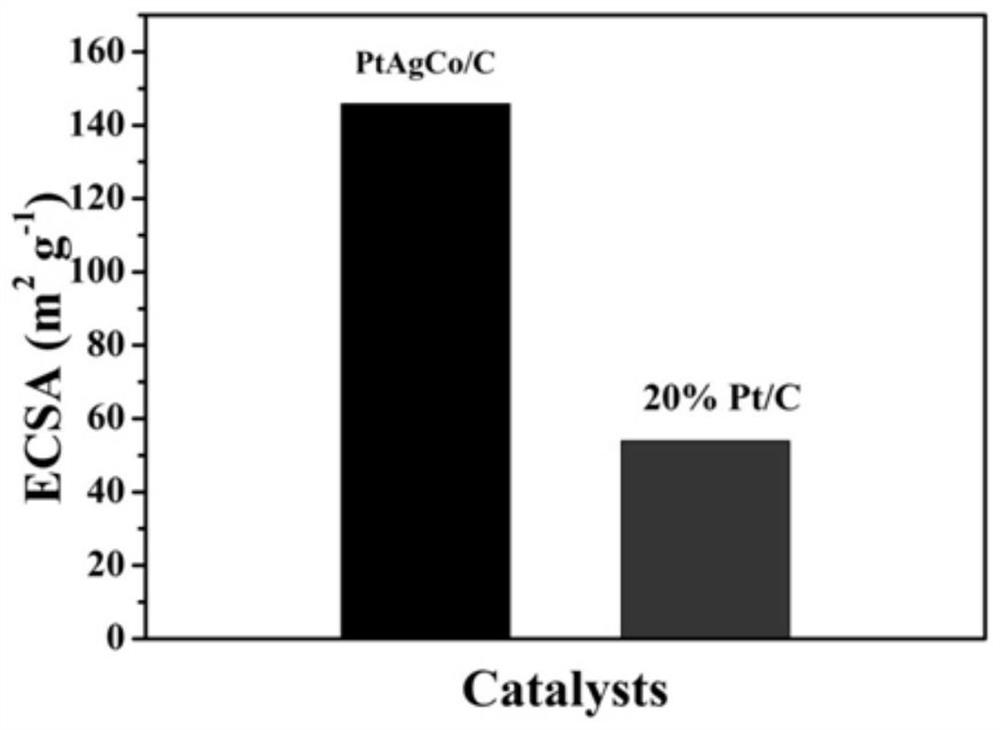
A kind of ptagco/c nano flower structure catalytic material and preparation method thereof and application as fuel cell catalyst
A technology of catalytic materials and nano-carbon materials, which is applied in the field of fuel cell catalysts, can solve problems such as failure to meet DOE standards, affect platinum active sites, and fast ECSA decay, and achieve improved load stability, good dispersion, and platinum content. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Configuration of metal precursor liquid:
[0041] According to the molar ratio of Pt:Ag:Co=1:1:1, the precursors chloroplatinic acid, silver nitrate, and cobalt acetate were respectively weighed and dissolved in the aqueous solution, and the ultrasonic dispersion was good, which was recorded as A solution;
[0042] Second, the configuration of the carrier solution:
[0043]Disperse the carbon carrier in ethanol, and ultrasonically disperse it evenly, and record it as B solution;
[0044] 3. Hydrothermal reaction
[0045] After mixing A and B solutions evenly, use ammonia solution to adjust the pH to weakly alkaline to obtain C solution (the volume ratio of water and ethanol is 1:1), which is placed in a hydrothermal reaction kettle, wherein the total mass fraction of metal ions is 0.01wt%, and the mass fraction of carbon carrier is 0.05wt%. Reaction at 140°C for 6h.
[0046] Four, washing and drying
[0047] 4) After the reaction was completed and cooled to room...
Embodiment 2
[0051] 1. Configuration of metal precursor liquid:
[0052] According to the molar ratio of Pt:Ag:Co=1:3:2, the precursors chloroplatinic acid, silver nitrate, and cobalt acetate were weighed and dissolved in the aqueous solution, and the ultrasonic dispersion was good, which was recorded as A solution;
[0053] Second, the configuration of the carrier solution:
[0054] Carbon support and SiO 2 The nanospheres were dispersed in ethanol, and ultrasonically dispersed to make them uniformly dispersed, which was recorded as B solution;
[0055] 3. Hydrothermal reaction
[0056] After mixing A and B solutions evenly, use ammonia solution to adjust the pH to weakly alkaline to obtain C solution (the volume ratio of water and ethanol is 1:1), which is placed in a hydrothermal reaction kettle, and the total mass fraction of metal ions in it is is 0.02wt%, and the mass fraction of the carbon carrier is 0.1wt%. Reaction at 160°C for 6h.
[0057] Four, washing and drying
[0058] ...
Embodiment 3
[0062] 1. Configuration of metal precursor liquid:
[0063] According to the molar ratio of Pt:Ag:Co=1:2:2, the precursors chloroplatinic acid, silver nitrate, and cobalt acetate were weighed and dissolved in the aqueous solution, and the ultrasonic dispersion was good, which was recorded as A solution;
[0064] Second, the configuration of the carrier solution:
[0065] Disperse the carbon carrier in ethanol, and ultrasonically disperse it evenly, and record it as B solution;
[0066] 3. Hydrothermal reaction
[0067] Mix A and B solutions uniformly to obtain liquid C (the volume ratio of water and ethanol is 1:1), which is placed in a hydrothermal reaction kettle, wherein the total mass fraction of metal ions is 0.02wt%, and the mass fraction of carbon support is 0.1 wt%. Reaction at 180°C for 6h.
[0068] Four, washing and drying
[0069] 4) After the reaction was completed and cooled to room temperature, the supernatant was discarded by centrifugation, and then poured...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com