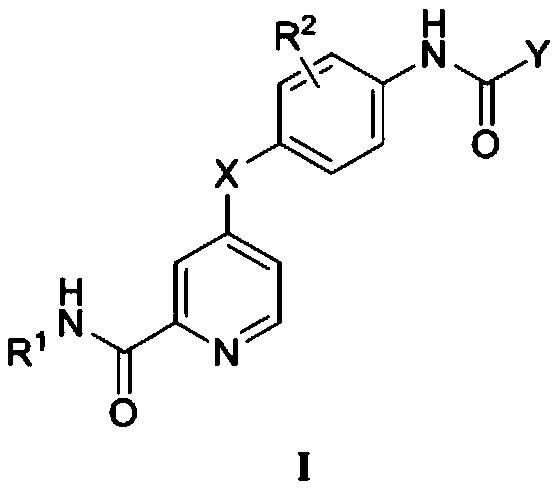
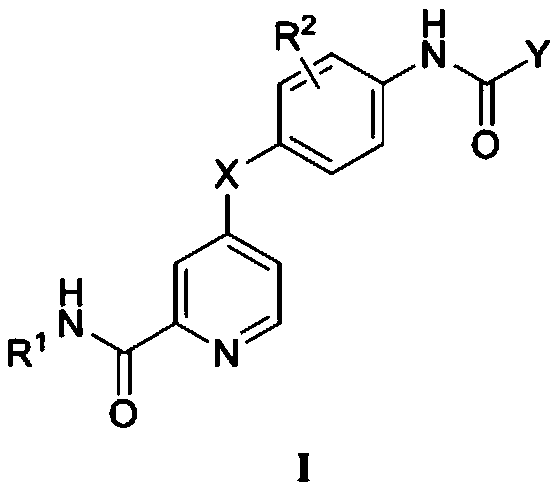
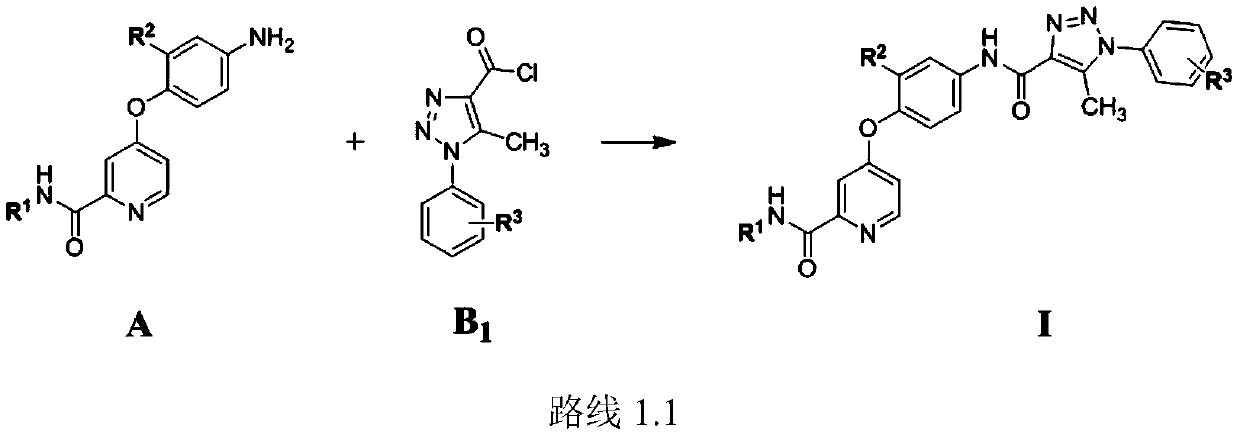
Picolinamide compound containing triazole or quinolinone structure and application of picolinamide compound
A technology of compound and pyridine, applied in medical preparations containing active ingredients, organic chemistry, drug combination, etc., can solve problems such as the absence of such compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Example 1 N-ethyl-4-(4-(5-(5-methyl-1-phenyl-1H-1,2,3-triazole-4-carboxamido)phenoxy)picolinylmethyl Amide
[0105] 1 H NMR (400MHz, DMSO-d 6 )δ10.75(s,1H),8.83(s,1H),8.51(d,J=5.3Hz,1H),8.03(s,1H),8.01(s,1H),7.63(s,5H), 7.41(s,1H),7.23(d,J=8.5Hz,2H),7.17(d,J=3.0Hz,1H),3.28(dd,J=13.3,6.6Hz,2H),2.59(s,3H ),1.09(t,J=7.0Hz,3H).
[0106] ESI-MS m / z:442.18
[0107] Step 1: Preparation of 4-chloropyridine chloride (b)
[0108] Add picolinic acid (10g, 0.081mol), thionyl chloride (50mL) for ultrasonic dissolution and NaBr (0.1g, 0.001mol) to a 150mL round-bottom flask, gradually raise the temperature to 85°C, and reflux for 24h. After the reaction solution was cooled to room temperature, the solvent was distilled off under reduced pressure, and an appropriate amount of toluene was added for use. A pale yellow liquid was obtained with a yield of about 97.5%.
[0109] Step 2: Preparation of ethyl 4-chloropicolinate (c)
[0110] Add dichloromethane (30mL), ethanol (16.43g...
Embodiment 2
[0130] Example 2 N-ethyl-4-(2-fluoro-4-(5-methyl-1-phenyl-1H-1,2,3-triazole-4-carboxamido)phenoxy)pyridine Formamide
[0131] 1 H NMR (400MHz, DMSO-d 6 )δ10.96(s,1H),8.86(s,1H),8.55(d,J=5.1Hz,1H),8.10(d,J=12.9Hz,1H),7.85(d,J=8.4Hz, 1H), 7.67(s, 5H), 7.47-7.40(m, 2H), 7.23(d, J=3.3Hz, 1H), 3.31-3.27(m, 2H), 2.59(s, 3H), 1.09(t ,J=6.8Hz,3H).
[0132] ESI-MS m / z:460.17
Embodiment 3
[0133] Example 3 N-ethyl-4-(4-(1-(4-fluorophenyl)-5-methyl-1H-1,2,3-triazole-4-carboxamido)phenoxy) picolinamide
[0134] 1 H NMR (400MHz, DMSO-d 6 )δ10.76(s,1H),8.84(s,1H),8.52(d,J=5.5Hz,1H),8.04(s,1H),8.02(s,1H),7.78(d,J=4.8 Hz,1H),7.76(d,J=4.7Hz,1H),7.53(d,J=8.6Hz,2H),7.42(d,J=1.9Hz,1H),7.24(d,J=8.8Hz, 2H), 7.19(d, J=2.3Hz, 1H), 3.29(dd, J=13.5, 6.7Hz, 2H), 2.59(s, 3H), 1.10(t, J=7.1Hz, 3H).
[0135] ESI-MS m / z:460.17
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com